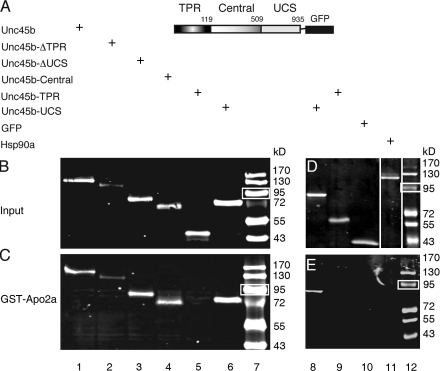
Figure 1.
Apo2a interacts with Unc45b but not Hsp90a in pull-down assays. (A) Schematic representation of the full-length Unc45b protein fused to GFP. Unc45b is composed of three domains: the TPR (amino acids 1–119), the central (amino acids 120–509), and the UCS (amino acid 910–935) domains. The intact Unc45b protein and its subdomains as well as Hsp90a were fused at their C terminus to GFP and used as input protein in the pull-down assays as indicated. (B) In vitro synthesized chimeric proteins visualized by Western blotting with anti-GFP antibody. (C) Full-length Unc45b-GFP fusion protein (lane 1) and deletion variants of Unc45b fused to GFP (TPR-deleted Unc45b [Unc45-ΔTPR] lane 2; UCS-deleted Unc45b [Unc45b-ΔUCS], lane 3; or single Unc45b domains fused to GFP [Unc45b-Central], lane 4; Unc45b-TPR, lane 5; Unc45b-UCS, lane 6) were pulled down with the GST-Apo2a fusion protein. With the exception of Unc45b-TPR (lane 5), all other chimeras were pulled down by GST-Apo2a fusion proteins (lanes 1–4 and 6). (D) Western blot of protein input of Hsp90a-GFP fusion (lane 11). Unc45b-UCS (lane 8), Unc45b-TPR (lane 9), and GFP (lane 10) were used as positive and negative controls, respectively, to test for an interaction of GST-Apo2a with Hsp90a-GFP. Note that relative to protein inputs in binding assays (C and E), 10-fold less protein was loaded onto the control gels shown in B and D. White lines indicate that intervening lanes have been spliced out. (E) Although Unc45b-UCS interacted with Apo2a (lane 8), neither the Unc45b-TPR fusion (lane 9), GFP alone (lane 10), nor Hsp90a-GFP (lane 11) were pulled down by GST-Apo2a. Thus, the interaction of Apo2a with Unc45b is specific. Lane 7 and 12 show protein markers. White boxes indicate 95 kD.