Figure 5.
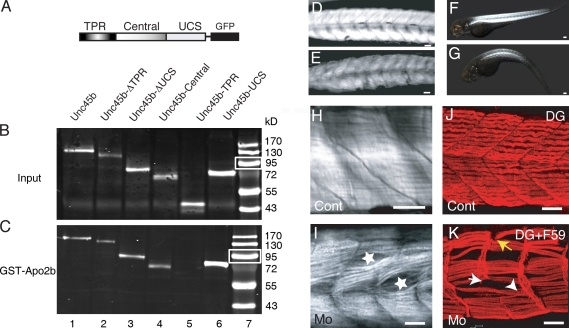
Apo2b interacts with Unc45b. (A) Schematic diagram of the Unc45b-GFP full-length and deletion proteins used in pull-down assays. (B and C) Western blot of in vitro translated input (B) and Unc45b-GFP proteins pulled down with GST-Apo2b (C). Fusion proteins were detected with an anti-GFP antibody. With the exception of Unc45b-TPR (lane 5), all the other fusions proteins were pulled down by Apo2b-GST (lanes 1–4 and 6), which indicates that both the central as well as the UCS domain can mediate the interaction with Apo2b. Lane 7 shows size markers. Note that relative to protein inputs in binding assays (B), 10-fold less protein was loaded onto the control gels shown in C. White boxes indicate 95 kD. (D and E) Control (D) and Mo(ATG)-apo2b (E)-injected embryos. Injection of the morpholino complementary to sequences surrounding the ATG of apo2b mRNA reduces the birefringence of the skeletal muscle. (F and G) Control (F) and Mo(UTR)-apo2b (G)-injected embryos. The morpholino complementary to the 5′ UTR of the apo2b mRNA reduces the birefringence of the skeletal muscle in a similar manner to the morpholino directed against the ATG of apo2b mRNA. The striking similarity of this phenotype to apo2a morphants suggests that the two Apo2 proteins do not act redundantly. (H and I) Mo(ATG)-apo2b morphants (I) but not controls (H) observed with DIC optics have cell-free spaces in the somites (I, stars). (J and K) Double immunohistochemistry with a slow muscle myosin antibody F59 and/or an α-dystroglycan (DG; J and K, respectively) antibody reveals extra-long intersomitic myofibers spanning two somites (K, arrowhead), detached myofibers, and defective myosepta in the morphants (K; white arrow, disrupted myosepta; yellow arrow, detached fibers). (I) Control. Bars: (D, E, and H–K) 30 µm; (F) 80 µm; (G) 100 µm.