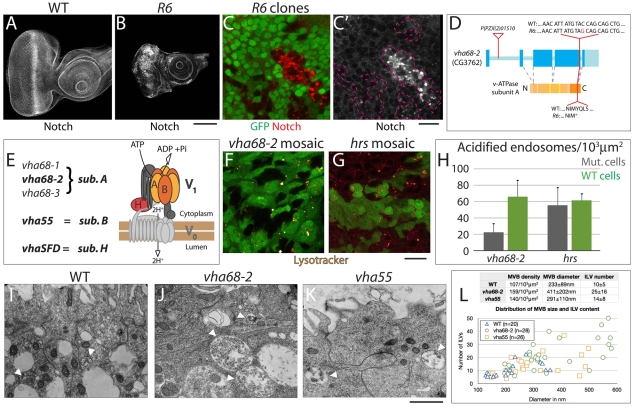
Fig. 1.
Altered Notch protein levels and endosomal acidification in Drosophila eye imaginal disc cells mutant for Vha68-2. (A,B) Eye imaginal discs stained to detect Notch. Compared with wild type (WT) (A), eye discs consisting predominantly of R6 mutant cells are smaller and show high levels of mislocalized Notch (B). (C,C′) Notch staining (red) of mosaic R6 eye discs; the absence of GFP expression marks the mutant tissue. Clones of R6 mutant cells (outlined in C′) display various degrees of Notch accumulation in intracellular puncta. (D) Drosophila Vha68-2 gene and protein organization with the locations and nature of mutant alleles shown. Exons and introns are shown in blue and light blue, respectively, and the protein domains and ATP-binding sites are shown in orange and yellow, respectively. The transposon l(2)01510 is inserted in the first intron, whereas the R6 mutation introduces a stop codon in the region encoding the ATP synthase α/β chain (dark orange). (E) List of genes that encode subunits A, B and H and schematic of subunit organization of the V-ATPase proton pump. Subunit A, which is encoded by Vha68-1, -2 and -3 (CG5075 – FlyBase) in Drosophila, is the ATP-binding subunit. (F,G) Lysotracker incorporation in cultured mosaic Vha68-2 (F) and Hrs (G) eye discs. Clones of mutant cells within WT tissue are marked by the absence of GFP expression. Compared with WT cells, Vha68-2 cells incorporate very little Lysotracker, indicating that endomembrane acidification is severely impaired. By contrast, clones of Hrs mutant cells incorporate similar levels of Lysotracker to WT cells. (H) Quantification of the experiment shown in F,G. (I-K) Transmission electron microscopy of WT eye discs (I) and of Vha68-2 (J) and Vha55 (K) completely mutant discs (see Materials and methods). A portion of two to three eye disc cells facing the peripodial membrane is shown in each micrograph. Multivesicular bodies (MVBs) are marked by arrowheads. (L) Quantification of the experiment shown in I-K. Average MVB density, diameter and internal luminal vesicle (ILV) content per genotype are presented in the table, and the distribution in size of representative individual MVBs and their ILV content are plotted. Compared with WT, Vha68-2 and Vha55 cells contain more and larger MVBs, which are filled with an higher number of ILVs. Extrapolation of volumes from the linear data suggests that Vha68-2 mutant cells contain up to three times more MVBs, and that these are up to five times larger than those of WT cells. Scale bars: 100 μm in A,B; 10 μm in C,C′,F,G; 1 μm in I-K.