Abstract
Vascular endothelial (VE)-cadherin is localized to the endothelial borders and the adherens junctions, which are regulated by changes in mitogen-activated protein (MAP) kinases, GTPases, and intracellular calcium. We previously showed that melanoma cells induce VE-cadherin disassembly through contact with human umbilical vein endothelial cells in coculture. However, the exact mechanism by which melanoma cells signal endothelial cells to induce VE-cadherin junction disassembly is not well understood. In this study, VE-cadherin junction disassembly was further examined under fluorescence microscopy. We found that melanoma-induced VE-cadherin junction disassembly and upregulation of p38 MAP kinase in endothelial cells is regulated by both soluble factors from melanomas, particularly interleukin (IL)-8, IL-6, and IL-1β, and through vascular cell adhesion molecule-1. Neutralizing melanoma-secreted soluble factors reduced endothelial gap formation. Endothelial cells transfected with MAP kinase kinase 6, a direct activator of p38 MAP kinase, increased VE-cadherin-mediated gap formation, facilitating melanoma transendothelial migration. In contrast, endothelial cells transfected with small-interfering RNA against p38 MAP kinase expression largely prevented melanoma transendothelial migration in Boyden chamber experiments. These findings indicate that p38 MAP kinase proteins regulate VE-cadherin junction disassembly, facilitating melanoma migration across endothelial cells.
Keywords: cytokines, chemokines, tumor conditioned medium, vascular cell adhesion molecule-1, mitogen-activated protein kinase, signaling, endothelial junctions, migration, vascular endothelial-cadherin
retraction of the endothelium during tumor cell extravasation occurs because of the disruption of intercellular channels or disassembly of vascular endothelial (VE)-cadherin homodimers that allow the passage of soluble proteins and cells (19, 22, 34). VE-cadherin is one such class of transmembrane endothelial junctions involved in regulation of the endothelial barrier playing an important role in angiogenesis, tumor metastasis, cell and protein signaling, and overall endothelial survival (3, 13, 22, 23). VE-cadherin localizes to the cell junctions and associates with α-catenin, β-catenin, plakoglobin, and p120 catenins via its cytoplasmic tails. Cadherin-mediated endothelial integrity is disrupted by phosphorylation of VE-cadherin tyrosine residues that result in decoupling of the p120 and β-catenin complex from VE-cadherin (1, 9). Previous studies show that disassembly of VE-cadherin homodimers is mediated by adhesion events (15, 37).
High expression levels of α4β1-integrin associated with highly metastatic melanoma cells is correlated with a marked increase in melanoma extravasation through endothelial layers (8, 11, 27). Although previous studies have focused on the effects of α4β1 and vascular cell adhesion molecule-1 (VCAM-1) interactions on metastasis and adhesion of melanoma cells to the endothelium (10, 22, 24, 38), we have found that these adhesion events lead to the disassembly of VE-cadherin, which facilitates melanoma transendothelial migration (12, 20). However, melanoma cells themselves secrete large amounts of soluble proteins, including interleukin (IL)-8, IL-6, IL-1β, and growth-regulated oncogene (Gro)-α. Melanoma cells with high metastatic potentials have been shown to secrete higher amounts of IL-8 (19, 28). Further studies showed that melanoma cells overexpressing and secreting higher levels of IL-8 show an increase in penetration of matrigel filters and that secretion of IL-8 can occur within 5 min in endothelial cells and platelets (14, 31, 35). These results were further supported by in vivo studies showing an overall decrease in tumorigenicity and metastasis when mice lacking CXCR2 (the receptor for IL-8) were injected with melanoma cells (28).
It is well established that p38 mitogen-activated protein (MAP) kinase activation plays a key role in the initial breakdown of VE-cadherin junctions to facilitate cell migration through the endothelium (18, 34). Van Wetering and colleagues (36) have shown that the VCAM-1 receptor on the endothelium induces intercellular gap formation through the Rho-like GTPase Rac1 signaling that results in activation of p38 MAP kinase proteins further downstream of Rac. However, in these studies, human umbilical vein endothelial cells (HUVECs) were prestimulateds with 10 ng/ml of IL-1β for 30 min before stimulation with anti-VCAM-1 antibodies, and the study focused on leukocyte transendothelial migration. The question remains as to whether melanoma cells trigger VE-cadherin disassembly primarily through cell-cell contact-mediated events or through soluble protein-receptor events. Furthermore, could these tumor-induced events modulate specific intracellular pathways in the endothelium leading to disassembly of VE-cadherin homodimers?
Previously, we have shown that adhesion of sialyl-Lewisx/a-negative melanoma cells to the endothelium is regulated by α4β1/VCAM-1 interactions under low shear flow conditions (12). It is therefore possible that melanoma-facilitated breakdown of VE-cadherin occurs through similar mechanisms as seen in leukocytes (15, 17).
Inhibition of phospholipase C (PLC) in endothelium was previously shown to decrease melanoma cell-induced VE-cadherin disassembly, indicating the importance of intracellular calcium pathways (20). However, it was also found that inhibiting phosphatidylinositol 3-kinase in HUVECs did not alter the breakdown of cadherin junctions when contacting melanoma cells (20). In light of these studies, we postulated that, while activation of these signaling molecules plays some role in facilitating melanoma metastasis, other downstream signaling molecules might play a more significant role in tumor-specific regulation of VE-cadherin junctions. Previous work has shown that VCAM-1/α4β1 interaction induced phosphorylation of p38 MAP kinase and VE-cadherin disassembly (1). We found that inhibiting p38 MAP kinase reduces VE-cadherin disassembly and subsequently decreases melanoma cell extravasation. The effects of inhibiting p38 using SB-220025 (a potent p38 inhibitor) on VE-cadherin disassembly and transendothelial migration was studied using immunofluorescence and Boyden chamber experiments. We found that soluble proteins, including IL-8, IL-6, IL-1β, and Gro-α released from melanoma cells, and VCAM-1/α4β1 interactions, regulate p38 MAP kinase pathways, which in turn regulate VE-cadherin junctions.
METHODS
Cells.
HUVECs were obtained from American Type Culture Collection (ATCC) (Manassas, VA) and maintained in F12-K medium with 10% FBS, 30 μg/ml of endothelial cell growth supplement, 50 μg/ml heparin (Mallinckrodt Baker), and 100 U/ml of penicillin-streptomycin (Biofluids). The Lu1205 melanoma cell line (kindly provided by Dr. Gavin P. Robertson, Pennsylvania State Hershey Medical Center, Hershey, PA) and A2058 cells (obtained from ATCC) were maintained in Dulbecco's modified Eagle's medium (DMEM; GIBCO) supplemented with 10% FBS and 100 U/ml of penicillin-streptomycin. WM35 melanoma cells (provided by Dr. Meenhard Herlyn, Wistar Institute, Philadelphia, PA) were maintained in Roswell Memorial Park Institute (RPMI) supplemented with 10% FBS and 100 U/ml of penicillin-streptomycin. All cells were maintained in a humidified incubator at 37°C and 5% CO2. Metastatic potentials were qualitatively assessed based on the listed references (Table 1).
Table 1.
Metastatic potentials for melanoma cells
Tumor conditioned medium.
Tumor cells (WM35, A2058, and Lu1205) were cultured in 75-cm2 flasks under growth conditions described above to 90–95% confluency, after which medium was aspirated and replaced with fresh 5 ml of RPMI (for WM35 cells) or DMEM (for A2058 and Lu1205 cells) with 2% FBS. The medium was then removed after a 24-h period of chosen tumor cell culture and centrifuged in 50-ml conical tubes at 1,500 rpm at 4°C for 5 min to remove any remaining cells.
Antibody neutralization and p38 inhibitor studies.
Before experiments, HUVECs were maintained in F-12K medium with 2% FBS without additional supplements mentioned above for 12 h at 37°C and 5% CO2. All experiments were carried out in F-12K medium with 2% FBS without additional supplements to ensure that signaling was not influenced by additional growth factors. For some studies, HUVEC monolayers were stimulated with anti-VCAM-1/F(ab)2 cross-linking antibodies (30 μg/ml). For studies where antibodies were used to neutralize cytokines, A2058 melanoma cells were incubated with either anti-IL-8 (15 ng/ml), anti-IL-6 (6 ng/ml), anti-IL-1β (0.1 ng/ml), or a combination of these antibodies for 30 min. A2058 melanoma cells with antibodies were then put in contact coculture with HUVECs for either 10, 45, or 90 min. VE-cadherin staining and assessment of gap formation were assessed as detailed elsewhere.
Before experiments, HUVECs were maintained in F-12K medium with 2% FBS without additional supplements mentioned above for 12 h at 37°C and 5% CO2. All experiments were carried out in F-12K medium with 2% FBS without additional supplements to ensure that signaling was not influenced by additional growth factors. For inhibitor studies, HUVECs were treated with 1 μM concentration of 5-(2-amino-4-pyrimidinyl)-4-(4-fluorophenyl)-1-(4-piperidinyl)imidazole (SB-220025) (Calbiochem; Gibbstown, NJ) for 30 min after which the inhibitor was washed out with F-12K medium containing 2% FBS, and then the HUVEC monolayer was stimulated by tumor conditioned medium (TCM), soluble recombinant proteins, or contact or noncontact coculture with melanoma cells.
Contact and noncontact coculture.
Before experiments, HUVECs were seeded on no. 1 cover slips and maintained in F-12K medium with 2% FBS without additional supplements mentioned above for 12 h at 37°C and 5% CO2. All experiments were carried out in F-12K medium with 2% FBS without additional supplements to ensure that signaling was not influenced by additional growth factors. For most experiments with contact coculture, 0.9 million melanoma cells were directly added to 0.3 million HUVECs on cover slips, and nonbound tumor cells were removed before staining (unless other concentrations are specified in the legends for Figs. 1–7). For noncontact coculture, 0.3 × 106 HUVECs were seeded on cover slips, and 0.9 × 106 A2058 melanoma cells were added to a Transwell insert (0.3-μm pore size) for 10, 45, and 90 min. Following coculture, Transwell inserts were removed, and VE-cadherin gap formation was assessed using fluorescence imaging.
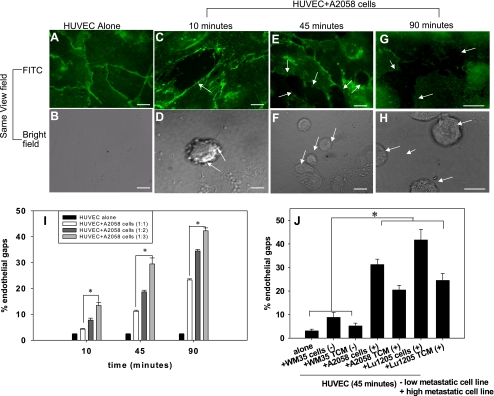
Fig. 1.
Melanoma cells induce vascular endothelial (VE)-cadherin disassembly. Bars in A–H, 5 μm. A: VE-cadherin junctions in human umbilical vein endothelial cells (HUVECs, 0.3 × 106 cells) without A2058 melanoma cells. HUVECs were fixed, permeabilized, and stained with anti-VE-cadherin monoclonal antibody (mAb) followed by Alexa 488. Representative fields were examined and show intact VE-cadherin junctions indicated by intact green fluorescent borders. B: in the same field of view as A, there are no tumor cells observed. C–H: disruption of VE-cadherin junctions after HUVECs were in direct contact with A2058 tumor cells for either 10, 45, or 90 min are shown using FITC. The same field of view captured under brightfield shows tumor cells in regions coinciding with gap formation. Arrows show disruption of VE-cadherin homodimers. I: HUVECs were cocultured with different concentrations of A2058 melanoma cells for 10, 45, and 90 min at 1:1, 1:2, or 1:3 ratios of HUVECs to A2058 cells. P values compare each experimental condition with %gap of HUVEC alone (*P < 0.05). J: HUVECs were cocultured in direct contact with tumor conditioned medium (TCM) or melanoma cells of increasing metastatic potential for 45 min. P values compare each experimental condition with %gap of HUVEC alone and WM35 cells/TCM (*P < 0.05). For all experiments, values are means ± SD.
Fluorescence imaging and analysis.
Before experiments, 25-mm cover slips were washed with PBS and then coated with fibronectin (1 μg/ml) and incubated at room temperature overnight under sterile conditions. Equal amounts of HUVECs were then grown to 95–99% confluency and in some cases treated with SB-220025 (a potent p38 MAP kinase inhibitor) for 30 min. HUVECs were cocultured with TCM, recombinant proteins, or A2058 melanoma cells in the presence or absence of SB-220025. Cells were then washed two times with PBS and fixed with 5% formaldehyde in PBS for 10 min. Following fixation, cover slips were washed two times with PBS, and cells were permeabilized with 0.3% Triton X-100 in PBS with 5% calf serum (CS) and 2% goat serum (GS). Cover slips were then incubated for 1 h and washed two times with PBS/CS/GS. Finally, each of the cover slips was incubated with either VE-cadherin, p38, or phospho-p38 antibody in 0.3% Triton X-100/PBS/CS/GS (dilution of 10:1,000) overnight at 4°C. Cover slips were then washed with PBS/5% CS/2% GS and treated with Alexa Fluor 488/520 goat anti-mouse IgG antibody (dilution of 1:1,000). The cover slips were then incubated at room temperature in the dark for 1 h and rinsed with PBS/5% CS/2% GS three times. To label the F-actin cytoskeleton, HUVECs were incubated with phalloidin conjugated with Alexa 546 in PBS/5% CS/2% GS (1:10 dilution) for 20 min at room temperature and rinsed with PBS/5% CS/2% GS three times before imaging under a Nikon fluorescence microscope. For each experimental condition, one cover slip was viewed under a ×100 objective, and a series of six images was taken of randomized fields of view. Each image was then analyzed using Image J software version 1.32 (23).
Analysis of gaps and disruption of VE-cadherin.
Disruption of VE-cadherin was identified from analysis of discontinuity of green fluorescence at VE-cadherin junctions between HUVECs. Gap area within disrupted VE-cadherin junctions was determined from six images (Fig. 1, C–H). Gap area was quantified as the ratio of pixels within all the gaps and the total number of pixels in one image (26, 30). The average percent endothelial gaps was calculated from six images and plotted as a function of time as shown in results.
Small-interfering RNA targeting p38α,β.
Small-interfering RNA (siRNA, 100 pmol) was introduced into 1.0 × 106 HUVECs using Dharmafect transfection reagent. Transfection efficiency was assessed using Western blot analysis as described below. HUVECs that were 80% confluent were transfected and assayed after 24–48 h. On-target pool siRNAs targeting p38 MAP kinase-α and p38 MAP kinase-β (Dharmacon, Lafayette, CO) were used for these studies along with nontargeting siRNAs (scrambled case). For a positive control, all stars siRNA targeting β-actin were used (Quiagen, Valencia, CA).
Western blots.
Equal amounts of whole cell lysates of HUVECs were prepared, and each condition was resuspended in cold lysis buffer [1 M Tris·HCl (pH 7.4), 5 M sodium chloride, 500 mM EDTA (pH 8.0), 1 M sodium vanadate, 1 M sodium fluoride, 1 M sodium pyrophosphate, 1.0% Nonidet P-40, 1.0 mM phenylmethylsulfonyl fluoride, 1% pepstatin, and phosphatase inhibitor] and incubated in ice for 30 min. Following incubation, cell lysates were centrifuged at 14,000 g for 5 min, and the supernatant was mixed with 1 M dithiothreitol and SDS buffer (4% SDS, 20% glycerol, 0.2% bromphenol blue, and 100 mM Tris base). Each well was loaded with an equivalent amount (3 μg/μl) of cell lysate. Western blot analysis on the samples was conducted following procedures previously described (20). Briefly, protein was transferred onto a 0.2-μm nitrocellulose membrane (Millipore, Billerica, MA). All Western blots were probed with primary antibodies against p38 MAP kinase (Cell Signaling Technologies), phosphorylated p38 MAP kinase (Cell Signaling Technologies), or β-actin (Santa Cruz Biotechnologies). All blots were reprobed with β-actin (Cell Signaling Technologies) to ensure equal loading during transfer of proteins. For all experiments, Western blots were scanned and quantified using Image J software. In experiments measuring p38 phosphorylation, HUVECs were cultured in normal medium or medium with 2% FBS without endothelial growth factors 12 h before experiments.
Enzyme-linked immunosorbent assay.
TCM collected from a 24-h culture of the respective tumor cell (WM35, A2058, and Lu1205) were stored at −20°C until enzyme-linked immunosorbent assay (ELISA) for individual cytokines was performed at the Pennsylvania State University General Clinical Research Center. Each 48-well plate was coated with the appropriate mouse anti-human capture antibody diluted in 0.1 M NaHCO3 (pH 8.2) at a final concentration of 2 μg/ml. The plates were incubated overnight at 4°C. The next day, each plate was washed three times in phosphate buffer solution containing 20% Tween 20 (PBS-T) and blocked for 2 h at room temperature using PBS with 1% BSA. Samples and standards were added at 100 μl/well and incubated overnight at 4°C. After washing plates with PBS-T, wells were incubated for 2 h at room temperature in detection antibody (concentration: 5 μg/ml). Each plate was washed with PBS-T and then conjugated with streptavidin peroxidase (concentration: 1 μg/ml) for 30 min at room temperature. Finally, each plate was subject to colorimetric analysis after incubating the plate at room temperature for 60–90 min in 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (Sigma Aldrich) substrate with 30% hydrogen peroxide. The plates were read at a wavelength of 405–415 nm using a microtiter plate reader.
Transfection with cDNAs.
Flag MAP kinase kinase (MKK) 6(glu) (Addgene plasmid 13518) and red fluorescent protein (mRFP; Addgene plasmid 13032) plasmid was kindly provided by Dr. Roger Davis and Dr. Doug Golenbock (University of Massachusetts Medical School, Worcester, MA). Clones were selected with ampicillin, and plasmid was extracted using a Qiagen Maxi Kit as per the manufacturer's instructions. Following DNA purification, transfection complexes were formed by adding 3 μg of MKK6(glu) and mRFP DNA to 25 μl of virofect reagent and 15 μl of targefect reagent (Targeting Systems, San Diego, CA). Transfection complexes were added to each well of HUVECs seeded on microslides in 1 ml of F12-K medium with 10% FBS. HUVEC responses were assayed using fluorescence microscopy and analysis 24–48 h posttransfection using Image J software. HUVECs were tested for MKK6(glu)/mRFP expression using Western blot analysis as described in previous sections.
Static cell migration assay.
For the static cell migration study, HUVECs were grown to confluency on fibronectin-coated polyvinylpyrrolidone-free polycarbonate filters (8 μm pore size; Neuroprobe). The wells on the bottom plate of the chamber were filled with HUVEC media with 2% FBS, and the middle 12 wells were filled with collagen type IV (concentration: 100 μg/ml in RPMI with 1% BSA) to act as a chemoattractant to melanoma cells (5, 29, 40). The filter was loaded on the top of the wells on the bottom chamber with HUVECs seeded on the opposite side, and then a top plate was screwed tightly to the static migration chamber. Melanoma cells were then loaded in wells on the top plate of the migration chamber in the middle 12 wells. After incubation at room temperature for 4 h, the number of melanoma cells migrating through the endothelial layer on the bottom of the filter was counted by staining the cells with Protocol Brand Hema 3 solution (Fisher Scientific) and counting stained cells using a inverted microscope (Diaphot 330; Nikon) with Image J software (23).
Statistical analysis.
Standard deviations for the percentage of endothelial gaps in all microscopic fields of view and Western blots represent three separate experiments that were performed in triplicate. Levels of significance of all experimental values were determined using a Student's t-test (Sigma Plot 8.0).
RESULTS
Melanoma cells induce VE-cadherin junction disassembly through cytokine and VCAM-1-mediated events.
Fluorescence imaging of HUVECs stained for VE-cadherin showed disruption of VE-cadherin junctions when cocultured with A2058 melanoma cells over 10, 45, and 90 min (Fig. 1). The breakdown of VE-cadherin was evident through the discontinuity of the green fluorescent line labeling the VE-cadherin junctions (Fig. 1, C, E, and G, arrows) compared with intact VE-cadherin junctions in the case of HUVECs cultured in control medium (Fig. 1, A and B). The corresponding brightfield images (Fig. 1, D, F, and H) show that the A2058 melanoma cells were located within the sites of gap formation labeled in Fig. 1, C, E, and G. These results show that highly metastatic melanoma cells induce breakdown of VE-cadherin junctions.
To determine if endothelial gap formation was dependant on the concentration of tumor cells, we measured gap formation when A2058 melanoma cells were in direct contact with HUVECs at a 1:1, 1:2, or 1:3 ratio for either 10, 45, or 90 min. Although melanoma cells in contact coculture with HUVECs in a 1:1 ratio resulted in more endothelial gap formation than the control case (Fig. 1I), the degree of gap formation increased dramatically when HUVECs were in contact with A2058 cells at either 1:2 or 1:3 ratios. These results show that endothelial gap formation increases linearly with the concentration of melanoma cells.
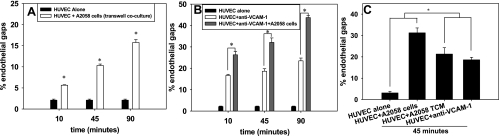
To determine if VE-cadherin disassembly was primarily mediated by soluble factor or receptor/ligand binding signals, HUVECs were brought in contact with TCM or melanoma cells with increasing metastatic potential for 45 min. Melanoma cells with increased metastatic potential, or TCM from those cells, showed a significant increase in ability to induce gap formation, indicating an increase in VE-cadherin disassembly (Fig. 1J). However, because TCM does not truly represent the “local concentration” of cytokines at the interface between tumor cells and endothelial cells, we used Transwell inserts to coculture HUVECs and A2058 melanoma cells to determine whether soluble proteins would still induce gap formation. While noncontact coculture of HUVECs and A2058 melanoma cells induces more gap formation compared with HUVECs alone (Fig. 2A), the degree of gap formation is higher when HUVECs are stimulated with TCM (Fig. 2C). Also, HUVECs stimulated with anti-VCAM-1 showed a significant increase in gap formation (Fig. 2B) similar to that seen in the presence of TCM. However, neither anti-VCAM-1 nor TCM induced the same degree of endothelial gaps as A2058 cells in coculture with HUVECs (Fig. 2C).
Fig. 2.
Endothelial gaps increase with metastatic potential of melanoma cells, which are mediated by both soluble factors and anti-vascular cell adhesion molecule-1 (VCAM-1). A: HUVECs were placed in indirect coculture with A2058 melanoma cells for 10, 45, and 90 min. The Transwell insert with melanoma cells was removed and %area of gap was assessed. P values compare each experimental condition with %gap of HUVEC alone (*P < 0.05). B: here, HUVECs were stimulated with anti-VCAM-1 for 45 min before adding A2058 melanoma cells. P values are comparing %endothelial gap of HUVEC + anti-VCAM-1 and HUVEC + anti-VCAM-1 + A2058 cells with %endothelial gap of HUVEC alone (*P < 0.05). C: HUVECs cocultured with TCM or anti-VCAM-1 significantly increase %gap formation compared with HUVECs alone, but less gap formation than A2058 cells cocultured with HUVECs. P values are comparing %endothelial gap of HUVEC + TCM and HUVEC + anti-VCAM-1 with %endothelial gap of HUVEC alone and HUVEC + A2058 cells (*P < 0.05). For all experiments, values are means ± SD.
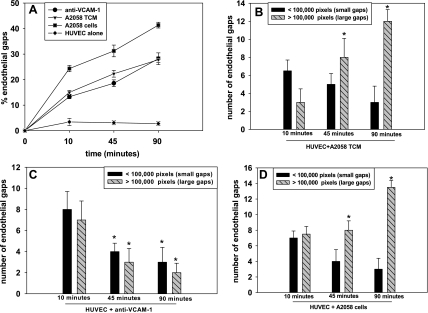
Because both anti-VCAM-1 and TCM induced a gradual increase in the percentage of endothelial gap formation over time (Fig. 3A), we determined how gap sizes were regulated. In the case of TCM, the degree of VE-cadherin disassembly and gap sizes increased gradually to >100,000 pixels or a 10-μm diameter by the 90-min time point (Fig. 3B). Figure 3C shows that anti-VCAM-1 induced larger gaps (sizes of 100,000 pixels or more) after 10 min, after which the number of large and small gaps decreased, indicating a decrease in VE-cadherin disassembly or closure of these gaps. These results suggest that VCAM-1 induces a transient VE-cadherin disassembly, while soluble proteins show a prolonged effect, thus enlarging existing gaps to allow the passage of melanoma cells, where the number of gaps after 90 min (Fig. 3D).
Fig. 3.
Endothelial gap sizes increase linearly over time. Experiments show the %endothelial gaps over time when A2058 TCM, anti-VCAM-1, or A2058 cells are cocultured with HUVECs over 10, 45, or 90 min. A: the %endothelial gaps as a function of time are plotted. B: the no. of gaps above or below an area of 100,000 pixels increased as a function of time; %endothelial gaps was plotted from data collected in A. P values are comparing the no. of large gaps in HUVEC + A2058 TCM after 45 and 90 min with no. of large gaps in HUVEC + A2058 TCM after 10 min (*P < 0.05). C: from data in A, the no. of endothelial gaps above or below an area of 100,000 pixels was plotted over time. P values are comparing the no. of large gaps in HUVEC + anti-VCAM-1 after 45 and 90 min with the number of large gaps in HUVEC + anti-VCAM-1 after 10 min (*P < 0.05). D: from data in A, the no. of small and large endothelial gaps was plotted as a function of time when HUVECs were cocultured with A2058 cells. P values are comparing the no. of large gaps in HUVEC + A2058 cells after 45 and 90 min with the no. of large gaps in HUVEC + A2058 cells after 10 min (*P < 0.05). All values are means ± SD.
Secretion of IL-8 and IL-1β by A2058 melanoma regulates VE-cadherin junction disassembly.
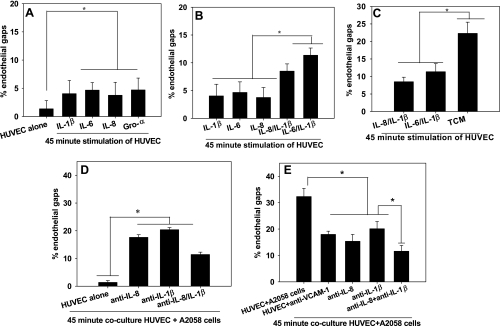
Cytokines present within TCM secreted by melanoma cells over 24 h were analyzed using a Raybiotech cytokine screen (data not shown). We found that several cytokines were secreted by melanoma cell types at high concentrations, including IL-8, IL-6, IL-1β, and Gro-α. These cytokines were further quantified using ELISA (Table 2). Clearly, highly metastatic melanoma cells (e.g., Lu1205) produce higher concentrations of these soluble cytokines compared with those of lesser metastatic potential (e.g., WM35). Stimulating HUVECs with recombinant forms of individual cytokines (at the same concentrations as that in A2058 TCM; see Table 2) showed little increase in the percentage of gap area (Fig. 4A). Combining cytokines in TCM, specifically IL-8 and IL-1β only had additive effects on VE-cadherin disassembly rather than being synergistic (Fig. 4, B and C).
Table 2.
Concentrations of cytokines secreted by melanoma over 24 h
| Melanoma Cell Line | IL-8 Concentration, ng/ml | IL-6 Concentration, ng/ml | IL-1β Concentration, ng/ml | Gro-α Concentration, ng/ml |
|---|---|---|---|---|
| Lu1205 | 24 | 23.2 | 5 | 0.08 |
| A2058 | 15 | 6 | 0.051 | 5.4 |
| WM35 | 0.084 | 0.078 | 0.00 | 0.001 |
IL, interleukin; Gro-α, growth-related oncogene-α.
Fig. 4.
A–C: interleukin (IL)-8 and IL-1β have additive rather than synergistic effects on VE-cadherin disassembly. A: the indicated cytokines induced VE-cadherin disassembly. P values are comparing %gap area of cytokines with %gap of HUVEC alone (*P < 0.05). B: stimulation of HUVECs with recombinant forms of IL-8 and IL-1β or IL-6 and IL-1β induced additive effects on endothelial gap formation. Concentrations of cytokines were based on TCM concentrations measured using ELISA (Table 2). P values compare %gap area of combinations of cytokines with %gap of HUVEC + IL-8, HUVEC + IL-6, and HUVEC + IL-1β. C: combinations of cytokines induced significantly less gap formation than TCM alone. P values are comparing %gap area of TCM with %gap of HUVEC + IL-8/IL-1β and HUVEC + IL-6/ IL-1β. D and E: anti-VCAM-1 and neutralization of both IL-8 and IL-1β dramatically reduces the breakdown of VE-cadherin. D: HUVECs were cocultured with A2058 cells + anti-IL-8, A2058 cells + anti-IL-1β, or A2058 cells + anti-IL-8 + anti-IL-1β. P values are comparing each experimental condition with %endothelial gap areas during HUVEC alone (*P < 0.05). E: using the same controls as in D, data were graphed to make comparisons between the effects of neutralizing individual and pairs of cytokines. HUVECs were cocultured with either A2058 cells, anti-VCAM-1, A2058 cells + anti-IL-8, A2058 cells + anti-IL-1β, or A2058 cells + anti-IL-8 + anti-IL-1β. Neutralization of both IL-8 and IL-1β dramatically decreased %endothelial gaps compared with HUVECs stimulated with anti-VCAM-1 or anti-IL-8 and anti-IL-1β alone. P values are comparing each experimental condition with %endothelial gap areas for HUVEC + anti-VCAM-1, HUVEC + A2058 cells + anti-IL-8, and HUVEC + A2058 cells + anti-IL-1β (*P < 0.05). Values for graphs and ELISA are means ± SD.
Because concentrations of cytokines in TCM are simply the bulk concentrations, HUVECs in direct contact with melanoma cells may sense much higher or lower local concentrations of cytokines within the cell-cell contact region than that found in TCM. We therefore specifically addressed whether IL-8 or IL-1β is involved in this response by neutralizing these cytokines secreted from melanoma cells during coculture with HUVECs. Neutralization of either IL-8 or IL-1β decreased VE-cadherin disassembly; however, the endothelial gaps were still comparable to that induced by anti-VCAM-1, and there are still more gaps compared with HUVECs alone (Fig. 4, D and E). However, simultaneous neutralization of IL-8 and IL-1β reduced the breakdown of VE-cadherin junctions (Fig. 4E). These results show the importance of these cytokines in melanoma-induced VE-cadherin disassembly in the presence of VCAM-1 interactions. Furthermore, using neutralization antibodies, we confirmed that IL-8 and IL-1β both play significant roles in the breakdown of VE-cadherin junctions.
Soluble protein and anti-VCAM-1 regulate p38 MAP kinase phosphorylation.
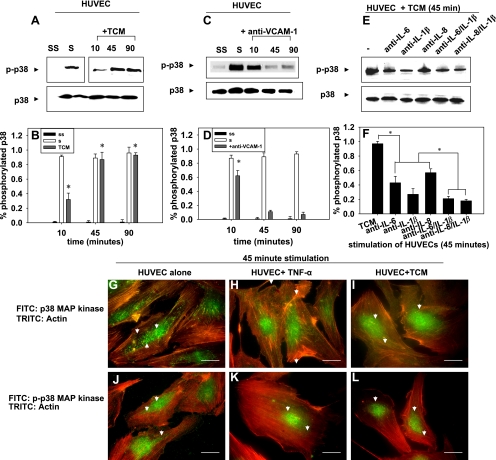
To determine whether the degree of VE-cadherin disassembly correlated with phosphorylation of p38 MAP kinase, soluble proteins from A2058 melanoma (TCM) were used to stimulate HUVECs for 10, 45, and 90 min. Lysates were subjected to SDS-PAGE to determine the levels of p38 phosphorylation. Stimulation of HUVECs with A2058 melanoma TCM upregulated p38 phosphorylation for up to 90 min (Fig. 5, A and B). On the other hand, stimulation of the HUVECs with anti-VCAM-1 upregulated p38 phosphorylation for 10 min before decreasing at 45 and 90 min (Fig. 5, C and D). Because neutralizing IL-8 and IL-1β decreased the degree of VE-cadherin disassembly, we tested whether neutralizing IL-8 and IL-1β affected p38 phosphorylation (Fig. 5, E and F). Neutralizing IL-8 and IL-1β did reduce p38 phosphorylation; however, this does not completely reject the possible involvement of other soluble proteins in this process. Because p38 phosphorylation varied when HUVECs were in the presence of TCM and anti-VCAM-1, we examined the spatial distribution of phosphorylated p38 to determine whether this is regulated during endothelial retraction.
Fig. 5.
A–F: soluble factors and anti-VCAM-1 regulate p38 phosphorylation. A: Western blots show p38 phosphorylation (p) when HUVECs are stimulated with A2058 TCM for 10, 45 min, and 90 min. The location of “S” and “SS” lanes have been moved for ease of interpretation but are from the same Western blot as other lanes. B: Western blots in A were quantified. P values are comparing TCM-stimulated case with the SS case. C: effects of anti-VCAM-1 on p38 phosphorylation in HUVECs over 10, 45, and 90 min. D: Western blots in C were quantified. P values are comparing TCM-stimulated case with the SS case. E: effects of neutralizing cytokines in TCM from A2058 cells for 45 min. Neutralizing antibodies (indicated) were respectively added in TCM before coculture with HUVECs. F: Western blots in E were quantified. P values are comparing TCM-stimulated case with cases where IL-8, IL-1β, IL-6, or a combination of these were neutralized in TCM and then added to HUVECs. Western blots are representative of 3 different experiments. Values are means ± SD. G–L: p38 mitogen-activated protein (MAP) kinase proteins translocate from the cytosol to the nucleus after phosphorylation (bars, 25 μm). HUVECs were fixed and stained with anti-p38 followed by Alexa 488-labeled secondary antibodies. The actin cytoskeleton was stained with phalloidin conjugated to Alexa 546. G: fluorescent images of unstimulated HUVECs. H: fluorescent images of HUVECs stimulated with recombinant tumor necrosis factor (TNF)-α for 45 min. I: HUVECs in contact with A2058 TCM for 45 min. J: fluorescent images of HUVECs fixed and stained with phospho-p38 followed by Alexa 488 secondary antibodies and phalloidin. K: fluorescent images of HUVECs stimulated with recombinant TNF-α for 45 min. L: fluorescent images of HUVECs in contact with A2058 TCM for 45 min. Images are representative of 3 separate experiments.
Imaging HUVECs before and after exposure to TCM or tumor necrosis factor (TNF)-α showed that total p38 was localized primarily in the cytosol, similar to that seen with HUVECs alone (Fig. 5, G–I). However, phosphorylated p38 was localized to the nucleus of endothelial cells with small amounts present in the cytosol in the case of HUVECs alone, most likely at the cytoskeleton or endothelial junctions (Fig. 5J). Increased localization of phosphorylated p38 within the nucleus was observed when HUVECs were in contact with TCM or TNF-α (Fig. 5, K and L). These results suggest that p38 may not regulate VE-cadherin junctions directly, but rather through interactions with other proteins.
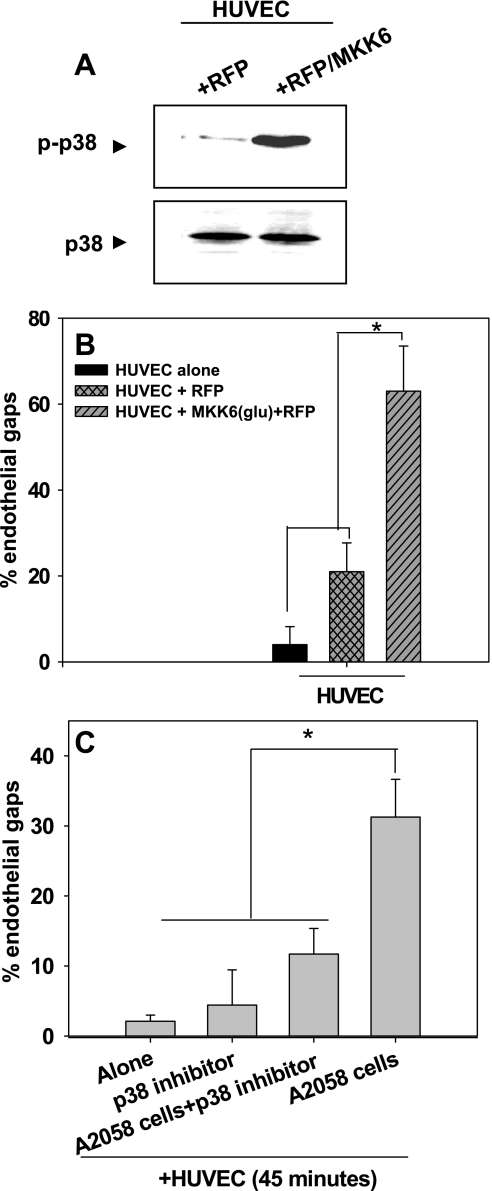
Because p38 MAP kinase activated in HUVECs via melanoma produced soluble mediators as well as anti-VCAM-1, we next determined whether constitutively active p38 would affect VE-cadherin disassembly. Transfection of MKK6 (an upstream activator of p38 MAP kinase) that leads to constitutively active p38 induced a high degree of gaps between endothelial cells compared with transfection of HUVECs with RFP alone (Fig. 6, A and B).
Fig. 6.
Overexpression of p38 increases disassembly of VE-cadherin homodimers to form endothelial gaps. A: transfection efficiency of HUVECs with red fluorescent protein (RFP) or MAP kinase kinase (MKK) 6(glu)/RFP was assessed using Western blots probed for either anti-p38 or anti-phospho-p38. Western blots are representative of three separate experiments. B: HUVECs were transfected with constitutively active MKK6(glu) fused with RFP to label transfected cells. Endothelial gap formation was assessed 36–48 h posttransfection. P values are comparing each experimental condition with %gap of HUVECs alone and HUVECs + RFP (*P < 0.05). C: inhibiting p38 phosphorylation prevents the disassembly of VE-cadherin homodimers. HUVECs were treated with SB-220025 for 30 min before they were cocultured with A2058 melanoma cells for 45 min. Values are means ± SD. P values are comparing each experimental condition with %gap of HUVEC + A2058 cells (*P < 0.05).
We further tested whether inhibiting p38 would affect the homotypic contacts formed by VE-cadherin. HUVECs pretreated with a potent p38 inhibitor (SB-220025) for 30 min and cocultured with A2058 melanoma cells showed significant decreases in gap formation compared with the control case (Fig. 6C).
p38 facilitates melanoma extravasation likely through the cytoskeleton.
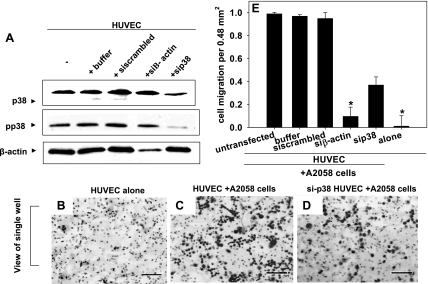
The functional role of p38 in endothelial cells during melanoma extravasation was also examined using siRNA approaches. siRNA-mediated knockdown of p38 (and thus phosphorylated p38) in HUVECs was confirmed using Western blotting (Fig. 7A). When p38 expression was knocked down, melanoma transendothelial migration after 4 h decreased significantly compared with the control (Fig. 7, B–D). In the controls, HUVECs did not migrate through the polycarbonate filters toward collagen IV (Fig. 7B). Melanoma extravasation through HUVECs decreased to nearly 40% when p38 was knocked out, compared with 98% of melanoma cells when p38 expression was at normal levels (Fig. 7E). These results show that p38 is not only important in the regulation of VE-cadherin junctions but also in overall tumor extravasation.
Fig. 7.
p38 MAP kinase activation facilitates melanoma extravasation. A: small-interfering (si) RNA knockdown of p38 (and thus pp38) was confirmed using Western blots. HUVECs transfected with siRNA against p38 (sip38), β-actin (siβ-actin), scrambled control, or buffer were lysed and subjected to SDS-PAGE to assess knockdown efficiency. B–D represent images of the underside of the polycarbonate filters from experiments using the Boyden chamber. Bars, 40 μm. B: HUVECs alone without A2058 cells. Bars, 40 μm C: HUVECs plus 50 μl of A2058 tumor cells (0.9 X 106 cells/ml) added to each of the 12 wells on the top plate of the Boyden chamber. D: HUVECs transfected with p38 siRNA plus A2058 tumor cells. The images of A2058 transmigration and Western blots are representative of 3 different experiments. E: average of migrated A2058 tumor cells for each experimental and control case. Values are means ± SD. P values are comparing each experimental condition with A2058 extravasation through untransfected HUVEC (*P < 0.05).
DISCUSSION
The interaction of bloodborne tumor cells with endothelial cells is a key step in facilitating melanoma metastasis (4, 30). However, research on drug therapies to treat such cancers has focused on single cell studies without considering the effects of tumor interactions with normal cell physiology. In these studies, A2058 melanoma cells were used to study VE-cadherin disassembly, since they are characterized by highly invasive capabilities and secrete high levels of soluble proteins, including growth factors and moderate levels of IL-8, IL-6, IL-1β, and Gro-α, and serve as an ideal system in studying metastasis (13, 21, 33). Lu1205 cells have even higher metastatic potential than A2058 or WM35 cells and secrete higher levels of IL-8, IL-6, IL-1β, and Gro-α, whereas WM35 cells exhibit the lowest metastatic potential and secrete the lowest levels of these cytokines (20, 33). Melanoma cells induce an increase in endothelial gap formation with increasing tumor cell concentration. In addition, melanoma cells with higher metastatic capabilities induce larger-sized gap areas that increase over time, corresponding to a higher degree of VE-cadherin disassembly. For instance, HUVECs in contact with Lu1205 cells for 10–45 min form gaps of average sizes of ∼100,000 pixels, whereas WM35 cells induce the formation of smaller gaps with an average size of ∼1,000 pixels. We show that anti-VCAM-1 initially induces the breakdown of VE-cadherin gap formation, with an increase in the number of gaps with a size of 100,000 pixels or greater after 10 min. However, after 45 and 90 min, there is a decrease in the number of larger gaps (>100,000 pixels), which shows that the gaps formed by anti-VCAM-1 are closing after 10 min. On the other hand, the release of soluble cytokines, including IL-8 and IL-1β, prolongs the time over which gaps remain open, which correlates with greater VE-cadherin disassembly. This phenomenon is shown by the gradual increase in the number of large gaps (size of 100,000 pixels or greater) over 90 min. The changes in the number of gaps correlate with phosphorylation of p38 MAP kinase where greater phosphorylation levels of p38 correlate with an increase in the number of gaps.
In this study, we focused on the specific soluble proteins that regulate disassembly of VE-cadherin homodimers to facilitate melanoma metastasis, since Transwell experiments confirmed that local concentrations of soluble proteins induce some disassembly of VE-cadherin, although not as much as TCM. In vivo studies have shown that VE-cadherin junction breakdown is an important event during melanoma metastasis (7, 25). These results show that the injection of nude mice with BV13 (anti-VE-cadherin antibody, which induces VE-cadherin disassembly) results in a fourfold increase in tumor metastasis and an increase in overall permeability of the endothelial layer (25). While these studies have focused on the in vivo aspects of VE-cadherin roles, our present in vitro studies provide the first evidence that shows the importance of soluble cytokines released from melanoma in regulating VE-cadherin junctions. In particular, we found IL-8 and IL-1β play a prominent role in soluble-mediated breakdown of VE-cadherin junctions. These results are consistent with previous in vivo studies that show that CXCR2−/− nude mice injected with melanoma cells result in a decrease in melanoma metastasis (28). Furthermore, we used TCM to show that soluble proteins alone are capable of facilitating the breakdown of these junctions showing that melanoma metastasis is not primarily mediated by adhesion events but rather that both VCAM-1 and soluble proteins control the temporal disassembly of VE-cadherin. We further tested whether adhesion events were capable of inducing the phosphorylation of p38 MAP kinase proteins by stimulating the HUVEC layer with anti-VCAM-1 functional antibodies. Previous studies have shown that the β1-subunit of α4β1 mediates binding to its ligand, VCAM-1 (2, 12). Furthermore, studies with A2058 melanoma cells have shown a dramatic decrease in invasiveness, adhesion, and migration of melanoma when the β1-subunit of α3β1-integrin of A2058 melanoma is blocked using functional antibodies (16). These results indicate the importance of α4β1 and VCAM-1 interactions in aggressive forms of melanoma metastasis (16). Our results show that VCAM-1 on endothelial cells induce the transient phosphorylation of p38 correlating with dramatic but early formation of large gaps (sizes of 100,000 pixels or more) in HUVECs. A size of 100,000 pixels corresponds to the size of A2058 and Lu1205 melanoma cells, which are ∼14 μm in diameter.
It is well known that p38 MAP kinase is regulated by IL-1β (42); furthermore, we have found that neutralizing IL-1β greatly reduces tumor-induced endothelial gap formation. Previous studies have found that IL-8 triggers a rise in intracellular calcium mediated by PLC (39). Our previous findings have found that inhibiting PLC greatly reduces tumor-induced endothelial gap formation (20). In the present studies, we therefore focused primarily on the importance of IL-8 and IL-1β in VE-cadherin disassembly; however, our results warrant further studies as to the exact role of IL-6 and Gro-α in melanoma metastasis, since these cytokines are upregulated during coculture of melanoma cells and HUVECs. Because the stimulation of HUVECs with TCM increased breakdown of endothelial junctions over a period of 45–90 min while anti-VCAM-1 only induced significant breakdown of VE-cadherin for short periods of time, secretion of soluble proteins induces larger gaps in the endothelium so that more metastatic melanoma cells may rapidly transmigrate between the endothelial cells before they are dislodged from the endothelium under physiological flow.
We investigated the effects of soluble proteins on endothelial signaling by elucidating the molecular events that regulate VE-cadherin junctions. Previous studies (34) have shown that the activation of Src kinases and VE-cadherin-mediated disassembly are required for the process of transendothelial migration in colon carcinoma (30). In particular, endothelial transmigration mediated by p38 MAP kinase cannot take place without phosphorylation-mediated formation of stress fibers of myosin light chains, in concert (34). The molecular mechanisms by which melanoma and colon cancer induce metastasis could be via similar signaling events. Consistent with previous findings (34), we find that inhibiting p38 MAP kinase decreases the degree of phosphorylation of VE-cadherin (data not shown). Because the phosphorylation state of VE-cadherin regulates the disassembly and assembly of these junctions, p38 MAP kinase must play a major role in regulation of the endothelial barrier (1). The importance of p38 MAP kinase in melanoma metastasis is strengthened by our studies where knock down of p38 MAP kinase in HUVECs resulted in a 60% decrease in melanoma migration through the HUVEC layer compared with the control case. Our results provide the first evidence that p38 MAP kinase activation plays a predominant role in overall endothelial permeability and tumor extravasation. Furthermore, we find that melanoma-produced soluble proteins dramatically affect the degree of p38 phosphorylation and VE-cadherin disassembly in HUVECs. Interestingly enough, neutralizing both IL-8 and IL-1β or IL-6 and IL-1β decreases p38 phosphorylation and VE-cadherin disassembly while melanoma cells contact HUVECs, suggesting the importance of these cytokines in breakdown of the endothelial barrier. In agreement with this conclusion, patients with highly aggressive melanomas have a marked increase in the secretion of IL-8, IL-6, and IL-1β (32, 41). Although IL-1β is normally secreted by white blood cells, a higher amount of IL-8, IL-6, and IL-1β is secreted by melanomas when in coculture with neutrophils (21). However, the role of these cytokines in tumor extravasation has yet to be elucidated. We have shown the importance of soluble proteins in p38 MAP kinase activation and that neutralizing these cytokines leads to a dramatic decrease in disassembly of VE-cadherin. However, because neutralization of IL-8, IL-6, and IL-1β did not completely abolish p38 phosphorylation, there may be other growth factors or cytokines released from melanoma that are capable of activating p38. In conclusion, our results support a role for cytokines and VCAM-1 binding in regulating p38 MAP kinase phosphorylation and the breakdown of VE-cadherin junctions.
GRANTS
This work was supported by National Institutes of Health Grants CA-125707 (C. Dong) and AI-065566 (A. August), National Science Foundation Grant CBET-0729091 (C. Dong), and National Health Research Institutes (Taiwan) Postdoctoral Fellowship Award PD9703 (H. Peng).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Roger Davis and Dr. Doug Golenbock for providing the MKK6(glu) and RFP plasmids for our experiments. Cell lines provided by Dr. Gavin P. Robertson (Pennsylvania State Hershey Medical Center) and Dr. Meenhard Herlyn (Wistar Institute) are greatly appreciated.
REFERENCES
- 1.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood 112: 2770–2779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo AG, Sánchez-Mateos P, Campanero MR, Martín-Padura I, Dejana E, Sánchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the beta 1 subunit. J Cell Biol 117: 659–670, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dejana E, Bazzoni G, Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res 252: 13–19, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Dong C, Slattery MJ, Liang S, Peng HH. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol Cell Biomech 2: 145–159, 2005 [PMC free article] [PubMed] [Google Scholar]
- 5.Dong C, Slattery MJ, Rank BM, You J. In vitro characterization and micromechanics of tumor cell chemotactic protrusion, locomotion, and extravasation. Ann Biomed Eng 30: 344–355, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 111: 1853–1865, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Garofalo A, Chirivi RGS, Foglieni C, Pigott R, Mortarini R, Martin-Padura I, Anichini A, Gearing AJ, Sanchez-Madrid F, Dejana E, Giavazzi R. Involvement of the very late antigen 4 integrin on melanoma in interleukin 1-augmented experimental metastases. Cancer Res 55: 414–419, 1995 [PubMed] [Google Scholar]
- 9.Gavard J. Breaking the VE-cadherin bonds. FEBS Lett 583: 1–6, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Giavazzi R, Foppolo M, Dossi R, Remuzzi A. Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J Clin Invest 92: 3038–3044, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer RH, Nicolson GL. Interactions of tumor cells with vascular endothelial cell monolayers: A model for metastatic invasion. Proc Natl Acad Sci USA 76: 5704–5708, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang S, Dong C. Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions. Am J Physiol Cell Physiol 295: C701–C707, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao F, Doody JF, Overholser J, Finnerty B, Bassi R, Wu Y, Dejana E, Kussie P, Bohlen P, Hicklin DJ. Selective targeting of angiogenic tumor vasculature by vascular endothelial-cadherin antibody inhibits tumor growth without affecting vascular permeability. Cancer Res 62: 2567–2575, 2002 [PubMed] [Google Scholar]
- 14.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 151: 1105–1113, 1997 [PMC free article] [PubMed] [Google Scholar]
- 15.Maschio AD, Zanetti A, Corada M, Rival Y, Ruco L, Lampugnani MG, Dejana E. Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-to-cell adherens junctions. J Cell Biol 135: 497–510, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melchiori A, Mortarini R, Carlone S, Marchisio PC, Anichini A, Noonan DM, Albini A. The alpha 3 beta 1 integrin is involved in melanoma cell migration and invasion. Exp Cell Res 219: 233–242, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Nicolson GL. Metastatic tumor cell attachment and invasion assay utilizing vascular endothelial cell monolayers. J Histochem Cytochem 30: 214–220, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Nwariaku FE, Chang J, Zhu X, Liu Z, Duffy SL, Halaihel NH, Terada L, Turnage RH. The role of p38 map kinase in tumor necrosis factor-induced redistribution of vascular endothelial cadherin and increased endothelial permeability. Shock 1: 82–85, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Okamoto M, Liu W, Luo Y, Tanaka A, Cai X, Norris DA, Dinarello CA, Fujita M. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1β. J Biol Chem 1–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng HH, Hodgson L, Henderson AJ, Dong C. Involvement of phospholipase C signaling in melanoma cell-induced endothelial junction disassembly. Front Biosci 10: 1597–1606, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng HH, Liang S, Henderson AJ, Dong C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp Cell Res 313: 551–559, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi J, Chen N, Wang J, Siu CH. Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway. Mol Biol Cell 16: 4386–4397, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasband W. Image J Bethesda, MD: National Institutes of Health, 1997 [Google Scholar]
- 24.Sandig M, Voura EB, Kalnins VI, Siu CH. Role of cadherins in the transendothelial migration of melanoma cells in culture. Cell Motil Cytoskeleton 38: 351–364, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Berse B, Jackman RW, Dvorak AM, Dvorak HF. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 12: 303–324, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Serck-Hanssen G, Blois A, Srebro B, Mandala M, Corti A, Helle KB. The chromogranin A peptide vasostatin-I inhibits gap formation and signal transduction mediated by inflammatory agents in cultured bovine pulmonary and coronary arterial endothelial cells. Regul Pept 135: 78–84, 2006 [DOI] [PubMed] [Google Scholar]
- 26a.Sharma A, Tran MA, Liang S, Sharma AK, Amin A, Smith CD, Dong C, Robertson GP. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res 66: 8200–8209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci USA 99: 9462–9467, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S, Varney M, Singh RK. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res 69: 411–415, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol 288: C831–C839, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens T, Moore TM, Schaack J, Creighton JR, Cooper DM, Cioffi DL. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol 157: 1267–1278, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su SB, Mukaida N, Matsushima K. Rapid secretion of intracellular pre-stored interleukin-8 from rabbit platelets upon activation. J Leukocyte Biol 59: 420–426, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Sulkowska M, Famulski W, Wincewicz A, Moniuszko T, Kedra B, Koda M, Zalewski B, Baltaziak M, Sulkowski S. Levels of VE-cadherin increase independently of VEGF in preoperative sera of patients with colorectal cancer. Tumori 92: 67–71, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Todaro GJ, Fryling C, De Larco JE. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci USA 77: 5258–5262, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tremblay PL, Auger FA, Huot J. Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP kinases. Oncogene 25: 6563–6573, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med 188: 1751–1756, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol 285: C343–C352, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol 19: 8–15, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Voura EB, Sandig M, Siu CH. Cell-cell interactions during transendothelial migration of tumor cells. Microsc Res Tech 43: 265–275, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Wu D, LaRosa G, Simon M. G protein-coupled signal transduction pathways for interleukin-8. Science 261: 101–103, 1993 [DOI] [PubMed] [Google Scholar]
- 40.You J, Mastro AM, Dong C. Application of the dual micropipette technique to the measurement of tumor cell locomotion. Exp Cell Research 248: 160–171, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, Gorelik E, Lokshin AE. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res 13: 2422–2428, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Research 15: 11–18, 2005 [DOI] [PubMed] [Google Scholar]