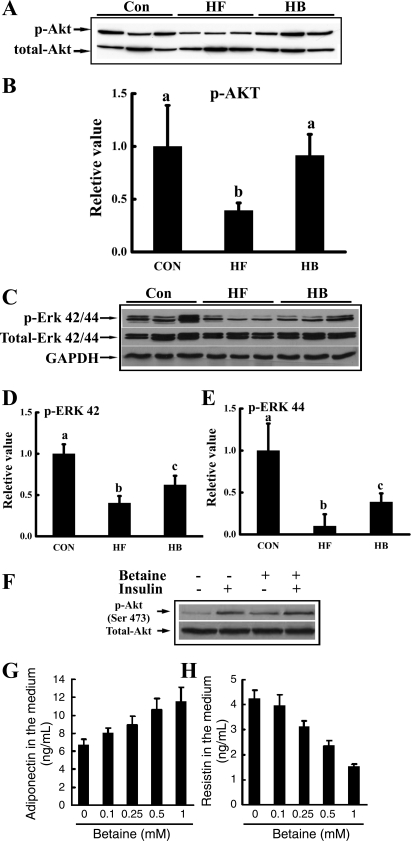
Fig. 5.
Betaine improved insulin signal transduction in adipose tissue both in vivo (A–E) and in vitro (F, G, and H). For in vivo studies, male C57BL/6 mice were fed a high-fat diet with/without betaine supplementation (1%) in the drinking water for 12 wk. Total protein extracts from epididymal fat pads were prepared thereafter. Protein (40 μg) was subjected to Western blot analysis for protein kinase B (Akt) and extracellular signal-regulated kinases (ERK) phosphorylation (p) using specific antibodies. A: betaine supplementation rescued adipose tissue Akt phosphorylation suppression induced by high-fat diet feeding. B: quantitation of Western blots of Akt phosphorylation levels in adipose tissues from different groups, normalized by corresponding total Akt expression levels (n = 8). C: betaine supplementation rescued ERK1/2 suppression induced by high-fat feeding. D and E: quantitation of Western blots of ERK42/44 phosphorylation levels in adipose tissues from different groups, normalized by corresponding total ERK42/44 expression levels (n = 8). Data are indicated as means ± SD. Bars with different letters differ significantly (P < 0.05). For in vitro investigation of insulin signaling, primary adipocytes from mouse fed a high-fat diet were isolated and cultured in a 6-well plate (1 × 106 cells/ml). Adipocytes were pretreated with 0.5 mM betaine for 2 h followed by stimulation with 10 nM insulin for 10 min. F: Akt phosphorylation status. For in vitro investigation of adipokine productions, primary adipocytes from mouse fed a high-fat diet were treated with different doses of betaine (0–1 mM) for 20 h. G: dose-response of adiponectin production. H: dose-response of resistin production. A total of three mice were used for the in vitro studies. Data are indicated as means ± SD from three independent batches of primary cultured high-fat diet adipocytes.