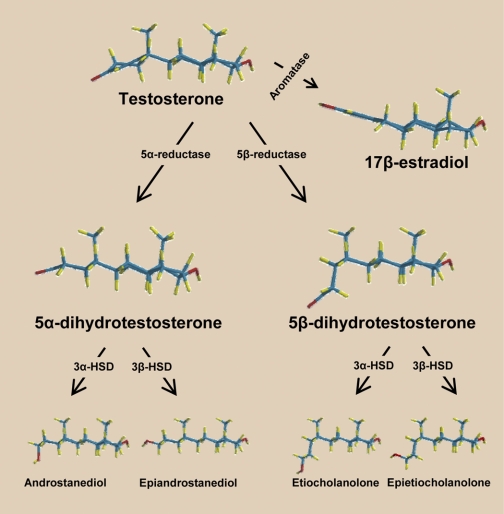
Fig. 2.
Metabolic pathways of androgens. Tes can be bioconverted into 17β-estradiol via the enzyme P-450-aromatase or into its immediate 5-reduced dihydro-metabolites: 5α-DHT (via the enzyme 5α-reductase) and 5β-DHT (via the enzyme 5-β-reductase). Subsequently, these dihydro-androgens undergo a 3α- or 3β-hydroxylation via the enzymes 3α- or 3β-hydroxysteroid dehydrogenase (HSD) to produce the tetrahydro-androgens (3α,5α-; 3β,5α-; 3α,5β-; and 3β,5β-reduced metabolites). Note the 3-dimensional conformation of the androgen molecules: Δ4,3-keto structure (Tes), 5α/trans-conformation (5α-reduced metabolites), and 5β/cis-conformation (5β-reduced metabolites). These molecular conformations reveal that minor changes in the orientation of C5 in the A-ring can result in major changes in the efficacy and potency of nongenomic vascular effects of the androgen molecule (e.g., 5α-DHT vs. 5β-DHT; see text for details).