Abstract
Reduced glutathione (GSH) is an intracellular molecule essential for many aspects of cell physiology and defense. Determination of GSH has been used to identify potential anti-cancer drugs and for the assessment of drug toxicity via generation of oxidative stress. The described protocol was designed to modify existing protocols for the fluorescent detection of intracellular GSH in a high throughput 96-well microplate format. Dibromobimane was used to label intracellular GSH, and an additional dye, Hoechst 33342 was used to measure cell density for data normalization. Cell density curves were performed using HEK 293T cells to determine the optimal starting cell density, (< 8.0 × 104 cells/well) for fluorescent analysis. Fluorescent dyes were also analyzed for compatibility and spectral overlap. The method was further validated by exposing HEK 293T cells to GSH modulating agents; tert-butylhydroquinone a potent inducer of GSH, and L-buthionine-(SR)-sulfoximine a potent inhibitor of GSH. This study provides a fast, simple method for the high throughput screening of GSH in a widely available 96-well format. It also addresses the pitfalls associated with fluorescent compounds in cell culture and proper data normalization.
Keywords: Glutathione, fluorescent spectroscopy, dibromobimane, Hoechst 33342, high throughput
Introduction
Glutathione (GSH) is a low molecular weight molecule considered to be the first line of cellular defense in mammalian cells (1). Composed of three amino acids, cysteine is the moiety capable of reacting with oxygen radicals and other electrophilic compounds. In addition to its antioxidant activities, GSH also participates in other processes including xenobiotic metabolism, gene regulation, intracellular signal transduction, and homeostasis of the cellular redox state (2,3). GSH is the most abundant intracellular non-protein thiol followed by cysteine; a substrate in the GSH synthetic pathway. Ultimately, changes in GSH can indicate a global antioxidant, or redox change in cells and has led researchers to monitor its levels; particularly in the context of induction of antioxidant systems, or assessment of oxidative stress.
Traditional methods for measuring GSH have involved biochemical assays dependent on the reaction of compounds with GSH such as 5,5’-dithio-bis-2-nitrobenzoic acid (4), or o-phthalaldehyde (5) that readily form chromophoric or fluorescent compounds, respectively, measurable by ultraviolet/visible or fluorescent spectroscopy. This method is considered reliable and highly quantitative; however, the preparation of tissue or cellular samples requires an investment of time and effort before measurement of GSH can be accomplished.
High performance liquid chromatography (HPLC) is an additional method for measuring GSH and is considered the most quantitative. HPLC utilizes a variety of systems including electrochemical (6) or fluorescent detection, the latter being a lengthier process requiring derivatization prior to analysis (7). Despite the quantitative aspect of HPLC, there is a large reliance on sample preparation to account for, and in some cases depending on sample size and run times, analysis can take 12 to 24 h to complete. In addition, analysis by HPLC can require increased costs for running and maintaining the instrument.
An alternatively accepted method for GSH measurement is to label intracellular thiols with fluorescent bimane compounds and analyze cells via fluorescent activated cell sorting (FACS) (8,9). FACS analysis of GSH does not demand as much time and can be simpler than biochemical methods; however, there are limitations. Sample preparation can require fewer steps than the aforementioned assays, yet ultimately the preparation requires the extraction and resuspension of cells before analysis can occur. Similar to HPLC, the availability of FACS instrumentation can also be problematic due to the initial costs and maintenance required for operation of the instrument. The sensitivity of FACS is another limitation to consider if the instrument is not equipped with the proper excitation or emission filters. Accurate measurement at optimal wavelengths is essential for FACS especially when using compounds with similar emission spectra.
Microplate readers are another technique for measuring fluorescent signals and can offer high throughput sample analysis by using a 96 or 384-well format. A large advantage of high throughput assays is that screening of several compounds or conditions can be performed in the same plate, thereby decreasing the variability encountered when separate cell culture dishes are analyzed. Fluorescent bimane dyes have also been previously characterized for use in the microplate format; however, an inherent problem with cell culture in the microplate format is that loss of cells can occur as a consequence of physical stressors such as media addition or removal. Thus, accuracy of the signal obtained could be skewed as a result of cell loss. The presence of an additional dye can be used in order to normalize each well to the cellular density actually contained therein, and allow for a more accurate assessment of the target endpoint.
This work describes a modification to previously published protocols for measuring GSH. It is a method designed for a high throughput assay to measure cellular GSH levels using 96-well microplates and a bimane compound, dibromobimane, that is fluorescently activated upon conjugation with thiol groups. The method is performed in combination with a viable nuclear stain, Hoechst 33342. Hoechst 33342 staining allows GSH levels to be normalized to the cell density contained in the well, thereby providing a more accurate value. Along with the high throughput capacity of this assay, there is the benefit of a shorter time interval between addition of target and reference compounds, and subsequent fluorescent measurement. By reducing time intervals, there is a decreased chance of non-specific labeling to occur, which decreases the potential for increased signal artifacts.
Materials & Methods
Cell culture
HEK 293T cells purchased from American Type Culture Collection were grown in flat bottomed, tissue culture treated 96-well microplates (Costar, Corning Inc., Corning, NY, USA) at 37° C, 5% CO2 under the following media conditions; 1X Dulbecco’s Modification of Eagle’s Medium with 4.5 g/L glucose and L-glutamine, 10% fetal bovine serum, 1 mM sodium pyruvate, 1X non-essential amino acids, 1000 international units penicillin – 1 mg/mL streptomycin, 50 µg/mL gentamicin sulfate; all media reagents were purchased from CellGro® technologies (Mediatec Inc., Herndon, VA, USA). HEK 293T cells were plated at starting densities between 5.0 × 103 – 2.0 × 105 per well, (n = 6 wells per cell density) in a final volume of 200 µL media and grown for a period of 16 hr.
Nuclear & GSH labeling
Media containing 1.5 µg/mL Hoechst 33342 (Molecular Probes, Carlsbad, CA, USA) was gently added at a volume of 100 µL to each well yielding a final concentration of 0.5 µg/mL Hoechst 33342/well and incubated for 30 min at 37° C. Plates were then drained of media by inversion and gentle shaking; any residual media at this point was removed by blotting the plate on bench paper. A stock solution of 4 mM dibromobimane (CAS # 68654-25-1; Sigma-Aldrich, St. Louis, MO, USA) suspended in 100% sterile-filtered dimethyl sulphoxide (Sigma-Aldrich, St. Louis, MO, USA) was made immediately prior to Hoechst 33342 media removal and diluted to a final concentration of 40 µM in sterile phosphate buffered saline, pre-warmed to 37° C. The dibromobimane was gently added to a final volume of 200 µL/well and incubated at 37° C for 30 min. (9,10).
Fluorescent measurement
Fluorescence was measured using a SPECTRAmax© Gemini XS fluorescent plate reader (Molecular Devices, Sunnyvale, CA, USA) at the following wavelengths: [Hoechst 33342 Exλ = 340, Emλ = 450], [dibromobimane Exλ = 393, Emλ = 477]. Fluorescent values were quantified using SOFTmax® PRO version 3.1.2 software (Molecular Devices, Sunnyvale, CA, USA).
Raw fluorescent results reported as fluorescent arbitrary units (F.A.U.) were obtained by first subtracting the mean blank value from all wells [F.A.U.experimental − F.A.U.blank]. Final results for GSH levels were reported as the ratio of dibromobimane normalized to Hoechst 33342 [dibromobimane F.A.U./ Hoechst 33342 F.A.U.]. For the validation studies cells were grown as described above and exposed to GSH modulating compounds. Following a 24 hr exposure of either 25µM tert-butylhydroquinone (tBHQ) or 100 µM L-buthionine-(SR)-sulfoximine (BSO), cells were stained and measured for GSH content as described above.
Statistical analysis
Statistical analysis involved the use of one-way ANOVA with student Newman-Keuls post-test for GSH modulating agents; Dunnet’s post-test was used for assessment of signal overlap between dibromobimane and Hoechst 33342.
Results & Discussion
Cultures of HEK293T cells were set up in 96-well microplates in a range between 5.0 × 103 – 2.0 × 105 cells/well. The objective was to determine the response of Hoechst 33342 over a range of cell densities. As seen in Figure 1A and Table 1, the Hoechst signal showed a linear response up to 8.0 × 104 cells/well. Cultures started at densities above that value had both an increase in the S.E.M (Figure 1A), and a decrease in the r2 value of the linear response (Table 1). Increasing the starting density from 8.0 × 104 to 1.0 × 105 cells/well results in an attenuation of r2 value from 0.9948 down to 0.7880, a trend that continued to yield a lower r2 with increased starting cell density.
Figure 1.
Measurement of fluorescent signal for Hoechst 33342 and dBBr in HEK293T cells as a function of increased starting cell density. (A) Hoechst 33342 signal at [λex340 nm, λem450 nm; open circles O], and at [λex 393 nm, λem450 nm; closed circles ●] in HEK293T cells (B) Dibromobimane signal at [λex 393 nm, λem 477 nm; open circles O], and at [λex340 nm, λem 477 nm; closed circles ●] in HEK293T cells. (C) Changes in dBBr fluorescence normalized to the Hoechst 33342 as a function of increasing cell density in HEK293T cells; dBBr fluorescent arbitrary units (F.A.U.) were normalized by the Hoechst 33342 F.A.U. of the same well. Values were calculated by the following formula; [dBBr F.A.U./Hoechst F.A.U.] and reported as mean ± SEM.
Table 1.
Linear Regression Summaries of Cell Density and Hoechst 33342 Response
| Hoechst 33342 Response to Cell Density Ex 340nm; Em 450nm | ||
|---|---|---|
| Cell Density | ||
| r2 | ||
| Range | Slope | |
| 5 to 20,000 | 1.37 × 10−02 | 0.9986 |
| 5 to 40,000 | 1.07 × 10−02 | 0.9846 |
| 5 to 60,000 | 1.05 × 10−02 | 0.9943 |
| 5 to 80,000 | 1.00 × 10−02 | 0.9948 |
| 5 to 100,000 | 6.87 × 10−03 | 0.7880 |
| 5 to 125,000 | 5.26 × 10−03 | 0.7127 |
| 5 to 150,000 | 3.85 × 10−03 | 0.5904 |
| 5 to 200,000 | 4.08 × 10−03 | 0.7366 |
n = 6 wells per density
This experiment sets up some important parameters for both cell type and assay conditions: (a) optimizing the starting cell density for the experiment; (b) determining the response of the experimental and reference stains; and (c) determining if any signal overlap exists between the experimental and reference stains.
As shown in Figure 1A and Table 1 the Hoechst 33342 dye is only effective up to a starting cell density of 8.0 × 104 cells/well for HEK 293T cells as indicated by the loss of linearity with higher starting cell densities. Cultures were then analyzed using the excitation wavelength for dibromobimane (393nm) and the emission wavelength for Hoechst 33342 (450nm).
Hoechst 33342 showed no signal when exposed to the dibromobimane excitation wavelength (393nm), thereby ensuring no signal overlap was occurring between the two stains at that particular excitation wavelength (Figure 1A). A similar scan was performed on the dibromobimane by using the excitation wavelength for Hoechst 33342 (340nm) and scanning at the emission wavelength for dibromobimane (477nm) to determine if the dibromobimane was creating a signal overlap while measuring the Hoechst 33342 dye (Figure 1B). As seen in Figure 1B, there is some response of dibromobimane at the Hoechst 33342 excitation wavelength (340nm). When analyzed in comparison to the mean value for the blank wells, the dibromobimane signal excited at the Hoechst 33342 wavelength (340nm) did not differ statistically until the cell density was higher than 8.0 × 104 cells/well, as determined by one-way ANOVA using a Dunnet’s post-hoc test. This finding further supports the idea that the maximum starting cell density should be no greater than 8.0 × 104 cells/well for this particular cell type based on (a) the loss of linearity of the Hoechst 33342 signal (Figure 1A), and (b) the increase in signal overlap between dyes at the higher cell densities (Figure 1B).
Contrary to the Hoechst 33342 in Figure 1A, dibromobimane is linear up to the maximum starting cell density of 2.0 × 105 cells/well (Figure 1B). This reinforces the importance of normalizing data, as demonstrated by the linear response of the dibromobimane. By measuring dibromobimane alone, it is possible that the results could yield inaccurate values if the cell density in the well does not remain constant throughout the experiment. A previous study demonstrated the use of GSH probes in a 96-well format; however, it was unclear what type of normalization, if any was performed (10). During these types of assays, the opportunity exists for loss of cell density due to cell death, or physical agitation during media addition or removal. Figure 1C shows the dibromobimane signal normalized by the Hoechst 33342 signal over increased starting cell densities (values calculated as F.A.U. dibromobimane/ F.A.U. Hoechst 33342). The range of starting cell densities between 6.0 × 104 cells/well and 1.5 × 105 cells/well yielded a balanced measurement of GSH levels. However, taking into account the Hoechst 33342 plot in Figure 1A, and the change in the r2 values indicated in Table 1, starting cell densities greater than 8.0 × 104 cells/well could be problematic.
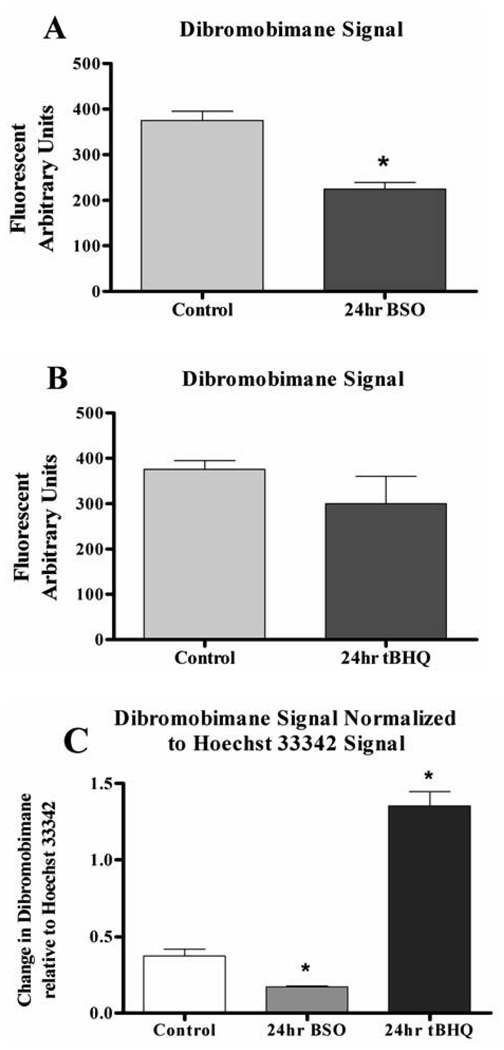
To further scrutinize this assay, cell cultures were set up at a starting density of 7.0 × 104 cells/well. Sixteen hrs later, cells were exposed to compounds shown to modulate cellular GSH levels; 25 µM tBHQ an inducer of GSH synthesis (11), and 100 µM BSO an inhibitor of GSH synthesis (12). Figure 2 demonstrates the importance of normalizing the dibromobimane fluorescent signal, rather than assuming equal cell density.
Figure 2.
Changes in GSH status of HEK293T cells exposed to GSH modulating agents. A) HEK293T cells exposed to 100µM BSO or B) 25µM tBHQ for 24 hours. C) Normalized values of dBBr from A and B, indicative of the GSH levels in the cells. All values are reported as mean ± SEM. * Indicates a significant fluorescent difference from control at p < .05.
Figure 2A shows the changes in dibromobimane signal in control, and cells exposed to BSO for 24 hrs. The data is displayed prior to Hoechst 33342 normalization and carries the assumption of equal starting cell density (Figure 2A). Although the results of this experiment display the expected outcome, (reduced GSH), the degree of reduction is less than levels reported using biochemical or HPLC methods (9,12). Normalization of the dibromobimane signal with the Hoechst 33342 signal yielded values more in accord with the published effects of BSO (Figure 2C). Treatment of cells with tBHQ has been reported to lead to an increase in levels of GSH; however, Figure 2B shows a reduction in GSH levels in the cells exposed to 25 mM tBHQ for 24 hrs. Once normalized to the Hoechst 33342 dye, the data showed increased GSH levels (Figure 2C); which were comparable with previous publications using tBHQ (11,13).
This method demonstrates a high throughput assay for determining changes in cellular GSH in a semi-quantitative manner. It combines the use of well-characterized fluorescent stains, ultimately allowing the measurement of the endpoint (GSH), and cell density in the same well of a standard microplate. This technique can be useful in screening compounds capable of modulating GSH; a strategy often used in the identification of potential chemotherapeutic compounds. It can also be used in the assessment of cytotoxicity for multiple compounds in one plate, as decreased GSH is often associated with conditions of oxidative stress and can therefore serve as a reliable marker of compromised cell status.
The assay can be utilized with immortalized cell lines, and more importantly with primary cell cultures (14), where limitations on the starting tissue and/or cell number may be an issue. The high throughput format allows for the screening of multiple fluorescent compounds, however, proper compound selection is an important parameter to consider due to signal overlap as a potential confounder for this type of assay. Finally, this assay addresses the pitfalls that may occur in 96-well microplate assays if the normalization to cell density is not performed.
Acknowledgments
This work was supported by the COBRE Program of the National Center for Research Resources (P20RRP20RR017670-04 and P20RR15583-07), and by NIA grant 1R15AG023604-01.
References
- 1.Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat Res. 1988;202:343–361. doi: 10.1016/0027-5107(88)90197-2. [DOI] [PubMed] [Google Scholar]
- 2.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 3.Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 4.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 5.Senft AP, Dalton TP, Shertzer HG. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal Biochem. 2000;280:80–86. doi: 10.1006/abio.2000.4498. [DOI] [PubMed] [Google Scholar]
- 6.Remiao F, Carmo H, Carvalho F, Bastos ML. Simultaneous determination of reduced and oxidized glutathione in freshly isolated rat hepatocytes and cardiomyocytes by HPLC with electrochemical detection. Biomed Chromatogr. 2000;14:468–473. doi: 10.1002/1099-0801(200011)14:7<468::AID-BMC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Martin J, White IN. Fluorimetric determination of oxidised and reduced glutathione in cells and tissues by high-performance liquid chromatography following derivatization with dansyl chloride. J Chromatogr. 1991;568:219–225. doi: 10.1016/0378-4347(91)80356-h. [DOI] [PubMed] [Google Scholar]
- 8.Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15:349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- 9.Horan AD, Chan CY, Pletcher CH, Menon C, Evans SM, Moore JS, Koch CJ. Analysis of tumor thiol concentrations: comparison of flow cytometric with chemical and biochemical techniques. Cytometry. 1997;29:76–82. [PubMed] [Google Scholar]
- 10.Sebastia J, Cristofol R, Martin M, Rodriguez-Farre E, Sanfeliu C. Evaluation of fluorescent dyes for measuring intracellular glutathione content in primary cultures of human neurons and neuroblastoma SH-SY5Y. Cytometry A. 2003;51:16–25. doi: 10.1002/cyto.a.10003. [DOI] [PubMed] [Google Scholar]
- 11.Hansen JM, Watson WH, Jones DP. Compartmentation of Nrf-2 redox control: regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol Sci. 2004;82:308–317. doi: 10.1093/toxsci/kfh231. [DOI] [PubMed] [Google Scholar]
- 12.Griffith OW. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982;257:13704–13712. [PubMed] [Google Scholar]
- 13.Eftekharpour E, Holmgren A, Juurlink BH. Thioredoxin reductase and glutathione synthesis is upregulated by t-butylhydroquinone in cortical astrocytes but not in cortical neurons. Glia. 2000;31:241–248. doi: 10.1002/1098-1136(200009)31:3<241::aid-glia50>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Tauskela JS, Hewitt K, Kang LP, Comas T, Gendron T, Hakim A, Hogan M, Durkin J, Morley P. Evaluation of glutathione-sensitive fluorescent dyes in cortical culture. Glia. 2000;30:329–341. [PubMed] [Google Scholar]