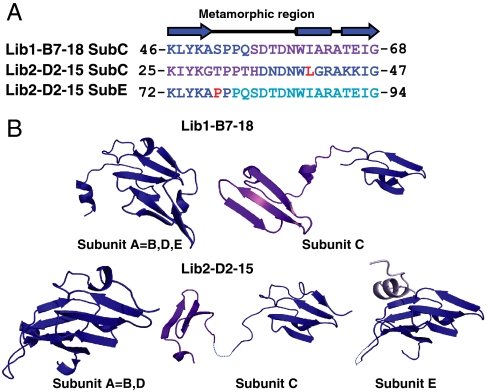
Fig. 3.
Structural metamorphism in the evolved oligomeric lectins. (A) Sequence alignment of the metamorphic region in subunit C of Lib1-B7-18 with the two metamorphic regions observed in subunits C and E of Lib2-D2-15. This region is a stretch of 23 amino acids that in wild-type tachylectin-2 comprises the third and the fourth strands of a blade and provides part of the sugar-binding site. Regions with wild-type-like structures are colored in blue and the alternative structure is colored according to Fig. 2B. Residues mutated during rounds of evolution are marked in red. (B) Structures of the intrapropeller subunits represented by subunit A in each variant and the interpropeller bridging subunits (subunits C and E in Lib2-D2-15, and subunit C in Lib1-B7-18). A short segment within the bridging subunit C in Lib2-D2-15 (within the linker connecting the two propellers), and a short loop in subunit E, appear to be disordered and are presented as dashed lines.