Fig. 2.
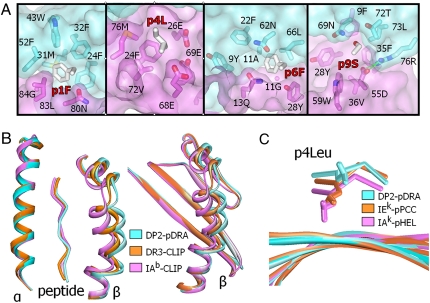
Unusual properties of the DP2 peptide-binding groove. (A) Details of the p1 (first panel), p4 (second panel), p6 (third panel), and p9 (last panel) binding pockets are shown. In each panel, the semitransparent water-accessible surfaces of the DP2 α1 (cyan) and β1 (magenta) domains are shown, as well as wireframe representations of the side chain of the pDRA amino acid at that position (CPK coloring) and the DP2 amino acids lining the pocket (CPK coloring, except for carbons: DP2 α1 carbons, dark cyan; and DP2 β1 carbons, dark magenta). (B) Three MHCII structures were overlaid on the basis of their α1 domains: DP2-pDRA (cyan), HLA-DR3 bound to the invariant chain CLIP peptide (PDB ID code 1A6A, orange) and mouse IAb bound to CLIP peptide (PDB ID code 1MUJ, magenta). (Left) MHCII α-helices as ribbons and the peptide from p1 to p9 as a CA stick. (Right) Full β1 domains as ribbons. (C) Three MHCII structure were overlaid on the basis of the four central β-strands of the floor of the peptide-binding groove: DP2-pDRA (cyan), mouse IEk bound to a peptide from pigeon cytochrome c (PCC; PDB ID code 1KTD, orange) and mouse IAk bound to a peptide from hen egg lysozyme (HEL; PDB ID code 1IAK). The figure shows the four central β-strands as ribbons, the peptide from p3 to p6 as a CA stick, and a wireframe representation of the side chain of the p4Leu in each structure.