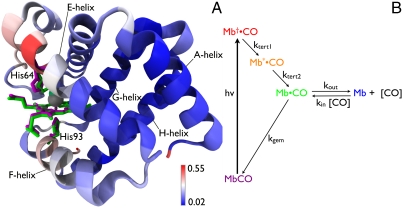
Fig. 1.
(A) Structural differences between MbCO and Mb. The heme, distal His64, and proximal His93 are rendered as licorice for both MbCO (magenta) and Mb (green). The backbone is rendered as ribbon and color coded according to the rmsd between these two structures. Structural alignment and rendering were carried out using the software VMD (33). (B) Five-state model for describing protein and ligand dynamics. Photolysis of MbCO produces Mb†•CO, an unrelaxed state with CO harbored in the primary docking site near the binding site. Tertiary structure relaxation is modeled in two steps, and produces Mb•CO with CO still harbored in the primary docking site. From this site, CO can either rebind geminately to recover MbCO, or can escape into the surrounding solvent or migrate into other internal cavities of the protein to produce Mb, which corresponds to deoxy myoglobin. Bimolecular CO binding regenerates the starting state, MbCO, and completes the fully reversible cycle. The color code for this five-state model is maintained throughout this manuscript.