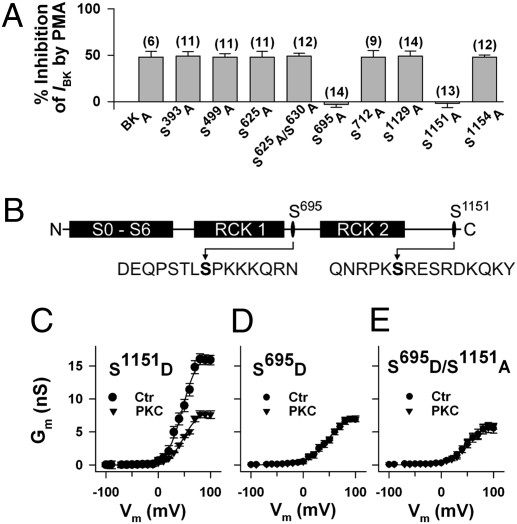
Fig. 2.
Inhibition of BK channel activity by PKC depends on serines 695 and 1151. (A) Mutation of the PKC phosphorylation sites Ser695 and Ser1151 to alanine abolished the inhibitory effect of the PKC activator phorbol 12-myristate 13 acetate (100 nM PMA) on BK channel whole-cell currents in transfected HEK293 cells. Percent inhibition of BK currents (IBK) at +80 mV is shown with number of cells in parentheses. The pipette solution contained 0.3 μM Ca2+. IBK before adding PMA was not significantly different between mutants (range: 121.2 ± 8.0 for S625A and 127.7 ± 12.3 pA pF−1 for S499A) and BKA (125.3 ± 13.1 pA pF−1). (B) Schematic representation of the BK channel with the positions of Ser695 and Ser1151 and their flanking regions, with the first methionine being in the context MANGG according to GenBank accession no. AAK54352.1. (C–E) Conductance-voltage relationships of mutants obtained from inside-out membrane patches before (Ctr) and after application of 30 nM PKCc (PKC). Means ± SEM of six (S1151D and S695D) and eight (S695D/S1151A) cells, respectively. Note, Gmax of channels inhibited by PKC resembles Gmax of phosphomimetic mutants. The intracellular (bath) Ca2+ concentration was 1 μM.