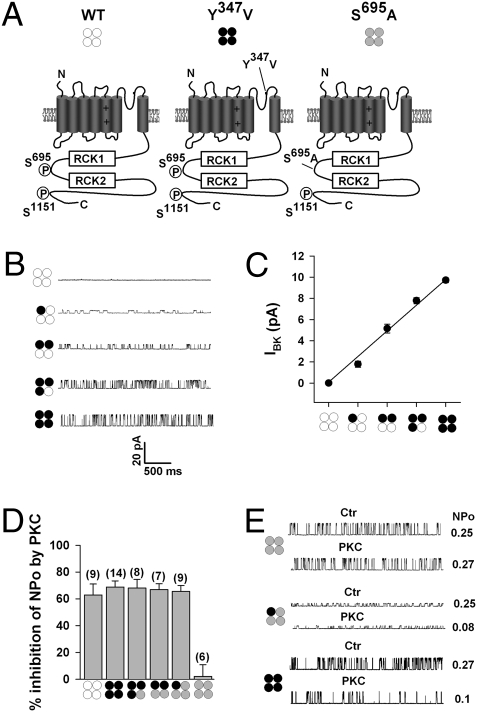
Fig. 3.
PKC inhibition of BK channels requires phosphorylation of a single α-subunit within the tetramer. (A) Schematic of the nonmutated BK channel α-subunit (WT, open circles), the TEA-insensitive Y347V α subunit (black circles), and the PKC site mutant S695A (gray circles). The critical PKC phosphorylation sites S695, located between regulator of K+ conductance (RCK) domains, and S1151 are indicated. Representative single-channel traces (B) and summary plot of single-channel amplitude (C) of BK channels in the presence of 2 mM TEA, assembled as heterotetramers of BK and Y347V α-subunits. Pictograms illustrate predicted channel stoichiometry. Recordings were at +40 mV in symmetrically high potassium (140 mM) from outside-out patches of transfected cells, means ± SEM, 6–14 patches per group. (D) Summary of effects of 30 nM PKCc on NPo by using BK-Y347V plus S695A heterotetramers with (3:1), (2:2), and (1:3) stoichiometry. For comparison, the PKC effect on homotetrameric WT−, Y347V−, and S695A− channels are also shown. Data from inside-out patches with symmetrically high potassium (140 mM) at +40 mV. With the exception of WT− and S695A homotetrameric channels, the recording pipette contained 2 mM TEA. Means ± SEM; n in parentheses. (E) Representative single records before (Ctr) and NPo after application of 30 nM PKCc (PKC) to channels composed as indicated by the pictograms. Recordings as in D. Intracellular free Ca2+ concentration was 0.3 μM.