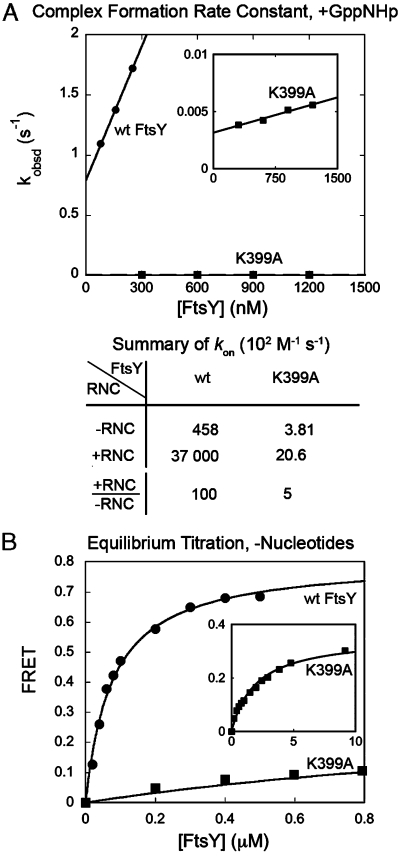
Fig. 5.
Mutation of FtsY–Lys399 diminishes the stimulatory effect of RNC on SRP–FtsY complex assembly. (A) Effect of FtsY–K399A on the rate constants of complex formation with cargo-loaded SRP. The inset shows the data with FtsY–K399A on an expanded scale. (B) Effect of FtsY–K399A on the equilibrium stability of the RNC–SRP–FtsY early targeting complex. The inset shows the data with FtsY–K399A on an expanded scale. Nonlinear fits of data gave Kd values and FRET end points of 76.5 nM and 0.72 for wild-type FtsY (•), and approximately 2 μM and 0.35 for mutant FtsY–K399A (▪).