Figure 3.
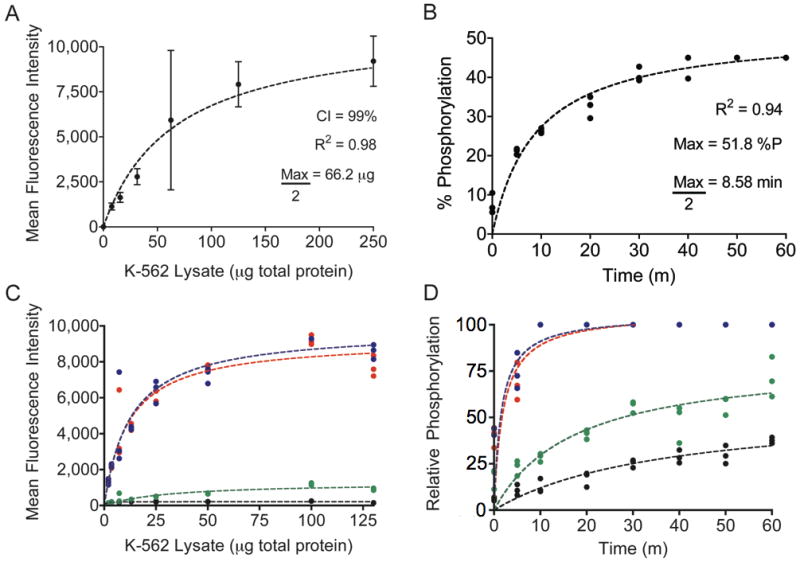
A, Abltide phosphorylation by K-562 cell lysates. Using mean fluorescence intensities and 99% confidence intervals from sampled beads, a series of only eight wells was sufficient to derive the central tendency of saturating kinase activity. B, reaction kinetics were monitored using 20 μg of total protein in K-562 lysates, less than half of the concentration necessary to obtain maximal Abltide phosphorylation. C, competitive substrate preference was determined by presenting four substrates immobilized on distinct Luminex beads: Abltide, immobilized via an amino-terminal cysteine (black) and via a carboxyl-terminal cysteine (green), and Abltide in tandem with p40, arranged with Abltide proximal (blue) or distal (red) to the immobilization site. Substrates that included p40 were phosphorylated to a greater extent using less cell lysates than required for Abltide alone (half-maximal phosphorylation with 11.6 μg (red) and 12.81 μg (blue) of total protein). Meanwhile Abltide reached a saturating fluorescence intensity of only 200 a.u. when presented in the company of substrates that included p40, compared to the 10,000 a.u. obtained when presented alone (A). D, Abltide was phosphorylated to a greater extent in a shorter amount of time when presented in tandem with p40 (blue and red), reaching half maximal phosphorylation in under two minutes. By comparison, Abltide (green, black) reached half maximal phosphorylation in over twice the time required when presented alone. In all cases, biological triplicates were best fit by hyperbolic curves.
