Figure 2.
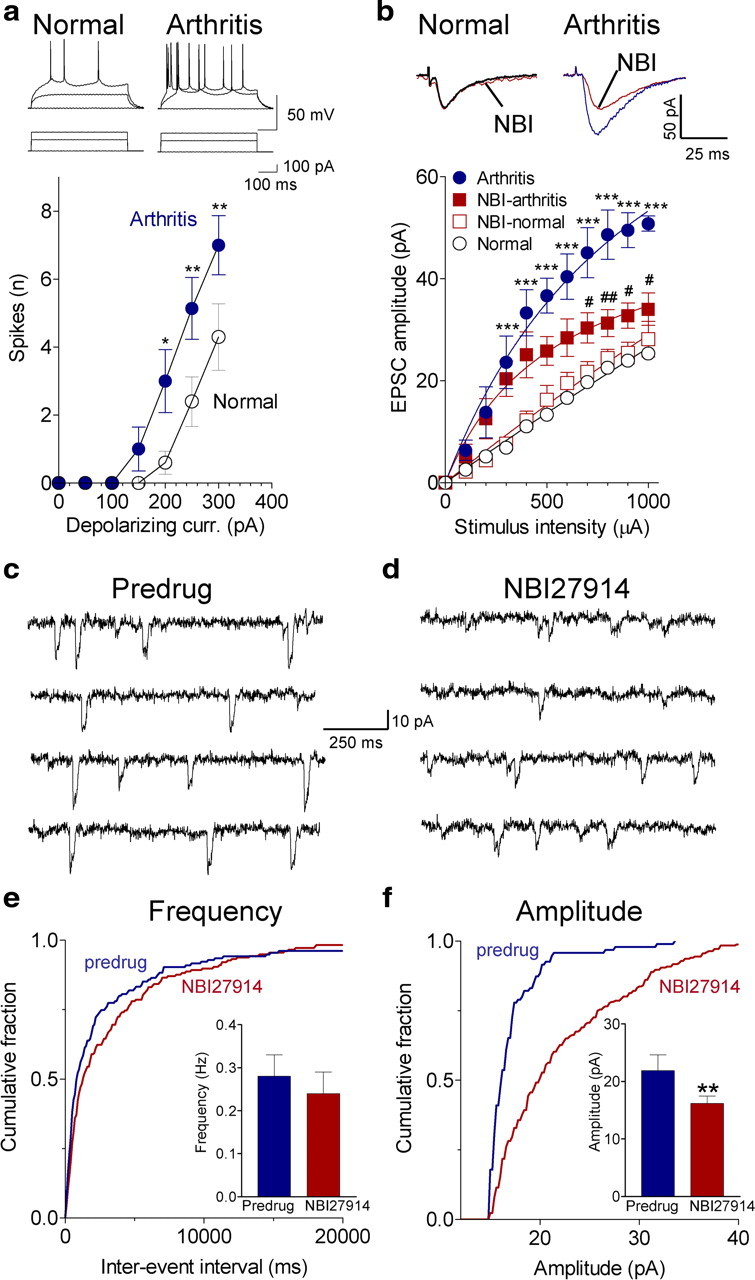
Pain-related changes in BLA neurons involves CRF1 receptors. a, Whole-cell current-clamp recordings of action potentials generated by intracellular current injections (500 ms) of increasing magnitude show significantly increased spike firing rate of BLA neurons in slices from arthritic rats (obtained 5–6 h after induction; n = 7 neurons) compared with normal controls (n = 10; Bonferroni's post hoc tests after two-way ANOVA; see Results). Symbols show mean ± SE number of spikes per 500 ms averaged for each sample of neurons. b, Whole-cell voltage-clamp recordings of monosynaptic EPSCs evoked at the LA–BLA synapse show that input–output function increased significantly (Bonferroni's post hoc tests after two-way ANOVA; see Results) in slices from arthritic rats (obtained 5–6 h after induction; n = 5 neurons) compared with control slices from normal rats (n = 5 neurons). NBI27914 (500 nm; 10–15 min) decreased EPSC amplitude in arthritis significantly (n = 5 neurons; Bonferroni's post hoc tests) but had no effect on normal transmission (n = 5 neurons). For each neuron, peak amplitudes of three to five EPSCs per stimulus intensity were averaged. Traces show averages of 8–10 evoked EPSCs. c, d, Original traces of miniature EPSCs recorded in TTX (1 μm) before and during application of NBI27914 (500 nm; 15 min) in a slice from an arthritic rat (5 h after induction). e, NBI27914 had no significant effect on cumulative interevent interval distribution and mean frequency of miniature EPSCs (n = 8 neurons; p > 0.05, paired t test; bar histograms). f, NBI27914 shifted cumulative amplitude distribution toward larger values, increasing mean amplitude significantly (n = 8 neurons; p < 0.01; bar histograms). e, f, Bar histograms shown mean ± SE before and during NBI27914. *,#p < 0.05, **,##p < 0.01, ***p < 0.001 (compared with normal and predrug, respectively).
