Figure 1.
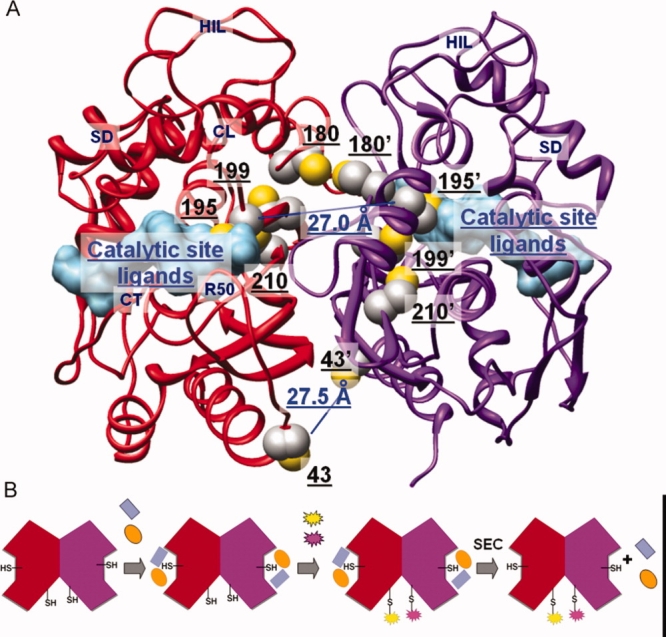
(A) Human thymidylate synthase structure (PDB: 1hvy). The two monomers (ribbon representation) are colored in red and purple, respectively. Ligands in the catalytic active site (surface representation) are colored in light blue. Side chains of cysteine residues (sphere representation) are colored by atom: C in gray, S in yellow. Distances between the S atoms of the C43/C43′ and C195/C195′ pairs are highlighted in blue. (B) Schematic walkthrough of the conjugation process: the two monomers of the dimeric protein are shown in red and purple; the two active-site ligands used to mask the active-site cysteine residues are in blue and orange, the fluorescent probes in yellow and magenta; SEC = Size exclusion chromatography. [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com.]
