Figure 1.
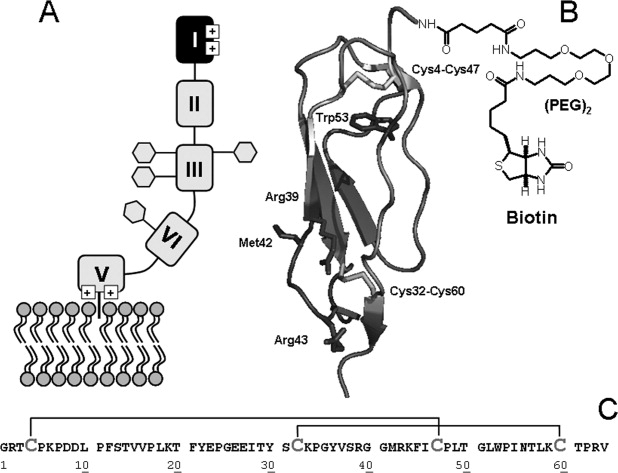
Structure and membrane binding of β2GpI. A: Schematic representation of full-length β2GpI interacting with phospholipid membranes. The four glycosylation sites in domain III and IV (i.e., Asn143, Asn164, Asn174, Asn243) are indicated by hexagons (adapted from Ref. 3). B: Schematic representation of the three-dimensional structure of N-DmI in the crystallographic structure of β2GpI (1qub).11 Disulfide bonds Cys4-Cys47 and Cys32-Cys60 and Trp53 are shown in stick together with Arg39, Met42, and Arg43 in the putatively primary antigenic epitope. Ribbon drawing of DmI was generated on the crystal structure of β2GpI using the software program ViewerPro 4.2 (Accelrys). C: Primary structure of the synthetic peptide DmI(1–64), as deduced from the amino acid sequence of full-length β2GpI.8 Cysteine residues are in gray and disulfide bonds are indicated by plain lines.
