Figure 5.
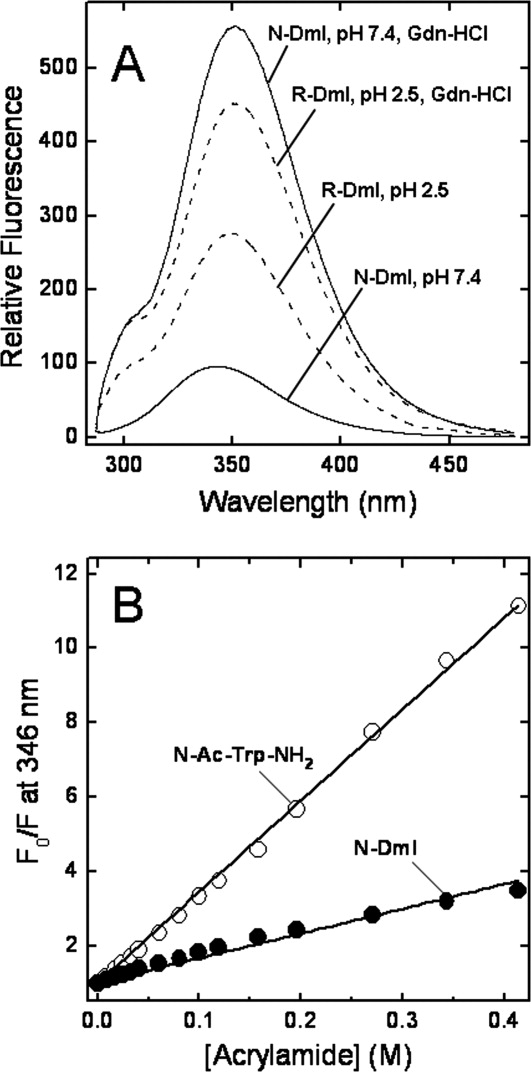
Fluorescence spectra and quenching experiments of DmI species. A: Fluorescence spectra of disulfide folded N-DmI (___) in 10 mM sodium phosphate buffer, pH 7.4, 0.15M NaCl in the presence or absence of 7M Gdn-HCl, as indicated; fluorescence spectra or the reduced species R-DmI (---) in 10 mM sodium phosphate buffer, pH 2.5, 0.15M NaCl in the presence or absence of 7M Gdn-HCl, as indicated. Protein samples (0.5 μM, 1.5 mL) were excited at 280 nm, and the spectra were recorded at 25°C ± 0.1°C. B: Acrylamide quenching of N-DmI fluorescence (•-•) and Nα-acetyl-Trp-NH2 (○-○). Fitting of data points to the Stern-Volmer equation (see Methods) yields Ksv values of 6.6 ± 0.7 and 25 ± 1.2 M−1 for N-DmI or Nα-acetyl-Trp-NH2.
