Abstract
There is compelling evidence that self reactive CD8+ T cells are a major factor in development and progression of Type 1 diabetes in animals and humans. Hence, great effort has been expended to define the specificity of autoimmune CD8+ T cells, and to alter their responses. Much work has focused on tolerization of T cells using proteins or peptides. A weakness in this approach is residual autoreactive T cells may be activated and exacerbate disease.
In this report we use a novel approach - toxin coupled MHC class I tetramers. Used for some time to identify antigen specific cells, here we use that same property to delete the antigen specific cells. We show saporin coupled tetramers can delete IGRP reactive T cells in vitro and in vivo. Sequence analysis of TCRβ chains of IGRP+ cells reveals the repertoire complexity in the islets is markedly decreased as NOD mice age and significantly altered in toxic tetramer treated NOD mice. Further tetramer+ T cells in the islets are almost completely deleted and surprisingly loss of tetramer+ T cells in the islets is long lasting. Finally, we show deletion at 8 weeks of age of IGRP+ CD8+ T cells, but not DMK or InsB reactive cells, significantly delays diabetes in NOD mice.
Introduction
Type 1 diabetes (T1D)2 is an autoimmune disease with a complex etiology. The disease in mice and humans is believed to be mediated by CD4+ and CD8+ T cells (1, 2). In both species, there is a progressive loss of insulin producing β cells in the islets of Langerhans. Genome-wide association studies have shown that polymorphism in the same genes of both species contribute to susceptibility to T1D, arguing that the fundamental processes are similar in mice and man (3). In NOD mice, the first evidence of insulitis is detected by 4 weeks of age and the majority of females develop frank diabetes by 20 weeks of age. The requirement of CD8+ T cells is well established. In the absence of CD8+ T cells, NOD mice do not develop T1D (4–6). Further, CD8+ T cells from diabetic mice are able to transfer disease (7). Finally a single CD8+ T cell clone derived from the TCR transgenic NOD 8.3 mouse is also able to transfer disease into immunocompromised NOD scid mice (8).
While it is clear that CD8+ T cells can cause islet destruction, the normal pathogenesis is likely more complex. There is good evidence that an autoimmune response to insulin is also required to develop diabetes in mice transgenic for the IGRP reactive NY8.3 T cell receptor (TCR) (9, 10). Further, mice that have an altered insulin gene that abolishes the major epitope recognized in NOD mice do not develop diabetes (11). Additional experiments to induce tolerance to the InsB epitope at an early age also blocked the development of T1D, although induction of tolerance to IGRP is also effective in preventing progression to T1D (12). In contrast, clinical trials attempting to induce oral tolerance to insulin have been ineffective (13, 14). Finally, treatment with anti-CD3 antibody has been effective in both mice and humans, but comes with significant side effects (including cytokine production) that limit its utility (15).
The use of MHC class I tetramers has revolutionized the study of CD8+ T cells (16). The ability to bind to antigen specific T cells could be used not only to identify antigen specific T cells, but also to carry toxins and radionuclide to the cells for either imaging or deletion (17–19). In our lab we have developed saporin coupled MHC class I tetramers to kill antigen specific T cells while sparing irrelevant T cells.
Several studies have shown that the repertoire of the islet infiltrating T cells changes over time (20–22). There has been little information about whether the changes in the repertoire observed are the cause or the effect of disease progression. Here we show that deletion of IGRP-specific CD8+ T cells changes the islet infiltrating T cell repertoire and prolongs the disease free interval of NOD mice.
Materials and Methods
Mice
NOD/ShiLtJ mice were purchased from Jackson Laboratories and housed in a specific pathogen–free laboratory animal facility that is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). NOD mice were used at 8–14 weeks of age. In all experiments, NOD mice were defined as diabetic if two consecutive weekly blood glucose measurements were greater than 250 mg/dl. Diabetes onset and incidence in unmanipulated mice is identical to that reported by Jackson Laboratories (data not shown).
NOD.Cg-Tg(TcraCl4,TcrbCl4)1Shrm/Tisch (NOD CL4) mice, which bear transgenic CD8+ T cells that react to the influenza peptide HA presented by H2Kd, were bred in-house (23). NOD.Cg-Tg(TcraTcrbNY8.3)1Pesa/DvsJ (NOD 8.3) mice, which bear transgenic CD8+ T cells that react to H2Kd-IGRP, and NOD.Cg-Prkdcscid (NOD scid) mice were purchased from Jackson Laboratories.
Isolation of Lymphocytes from Islets
Pancreata were perfused with a 2 mg/ml solution of collagenase P (Sigma), dissected and incubated at 37°C for 20 min. Islets were purified using a Ficoll PM 400 (Sigma) gradient, handpicked and counted then cultured overnight in RPMI 1640 containing 10% FBS and 4 ng/ml recombinant murine IL-2 (Invitrogen). Infiltrating lymphocytes were collected and filtered through a 40 µm nylon filter. Each sample constituted all of the islet cells isolated from an individual mouse.
Tetramer
MHC class I tetramers were prepared as previously described (24). Briefly, H2Kd or H2Dd and mouse β2-microglobulin were produced in E. coli and refolded with peptide (from GenScript) in vitro. Refolded peptide-MHC monomer was purified by HPLC, biotinylated using Biotin Protein Ligase, and assembled into tetramers by conjugation with UltraAvidin-PE (Leinco). Peptides used were NRP-V7, KYNKANVFL; IGRP 201–214, VYLKTNVFL; InsB 15–23 (G9V), LYLVCGERV; or DMK 138–146, FQDENYLYL
Toxic Tetramer Preparation and Injection
NRP-V7-H2Kd tetramers conjugated to saporin were prepared as previously described for gp33-H2Db tetramers (17). Eight week-old NOD female mice received 3 intravenous (i.v.) injections in the lateral tail vein, 6 d apart, beginning at 8 weeks of age. Injections contained 34 pmoles (4.36µg) of Kd-NRP-V7-SAP, Kd-IGRP-SAP, Kd-InsB-G9V–SAP or Db-DMK-SAP in 200µl of PBS. Control NOD female mice received three injections of 200µl PBS at the same time as the toxic tetramer injections. TCR analysis of CD8+ T cells in the spleen, pancreatic lymph nodes (PLN), and islets was carried out either at 21 d post-treatment or at the time of diabetes diagnosis or 54 weeks of age.
In Vitro and In Vivo Depletion of 8.3 and CL4 T Cells by Toxic Tetramer
As previously described, dissociated splenocytes from transgenic and wild-type NOD mice were either enriched for CD8+ T cells via negative selection, or depleted of CD8+ T cells via positive selection, using the appropriate immunomagnetic cocktails and LS columns on a QuadroMACS separator (Miltenyi Biotec, Sunnyvale, CA) (17). Enriched T cells were resuspended in PBS and injected i.v. via the lateral tail vein (2 ×105 – 5 × 106 / 200 µl / mouse). T cells were primed by intraperitoneal (i.p.) injection of IGRP206–214 peptide (100 µg) 2 h post-transfer. Tetramers were diluted in PBS and injected i.v. (22 pmol, (2.82µg) / 200 µl / mouse). To assay cell survival, peripheral blood was collected from the superficial temporal vein using a Goldenrod lancet (MEDIpoint, Inc., Mineola, NY). Lymphocytes were enriched over a Lympholyte-M gradient, stained with tetramers and antibodies for 1 h at 4°C, and analyzed on FACSCalibur flow cytometer (BD Biosciences, San Diego, CA).
Flow Cytometry and Single-cell Sorting
Lymphocytes from NOD mice were examined using flow cytometry. For sorting experiments, cells were stained with a cocktail of Alexa Fluor 488-anti-CD3 (Invitrogen), Pacific Blue-anti-CD4 (Invitrogen), APC-anti-CD8 (eBiosciences), PE-Cy7-anti-CD19 (BioLegends), and PE-ultraavidin(Leinco)-NRP-V7-Kd tetramer. For single-cell sorting, NRP-V7+CD3+CD8+ CD4−CD19− T cells were sorted by a MoFlo highspeed sorter (DakoCytomation) at 1 cell/well into a 96-well PCR plate (USA Scientific), each well containing 4 µl buffer of 0.5x PBS, 10 mM DTT, and 8 U RNaseOUT RNase inhibitor (Invitrogen Life Technologies). Plates were kept frozen at −20°C or −80°C. For tetramer-specific cell analyses on non-sorted samples, cells were stained with a cocktail of APC-Cy7-anti-CD3 (BD Biosciences, San Jose, CA), Pacific Orange-anti-CD4 (Invitrogen, Carlsbad, CA), PerCP-anti-CD8 (BD), Pacific Blue-anti-CD44 (Invitrogen), PE-Texas Red-anti-CD62 (Invitrogen), PE-Cy7-anti-CD19 (BioLegends, San Diego, CA) and PE-ultra-avidin (Lenco, St. Louis, MO)-NRP-V7-Kd tetramer. Antibody concentrations used were determined by preliminary titrations. Cells were analyzed on a Dako (Beckman-Coulter) Cyan-ADP(Colorado Springs, CO). “Minus one” controls were performed in each experiment to ensure that the fluorescence measured originated from the correct stain. Staining controls used syngeneic spleen cells. Flow cytometry data was analyzed using Summit 4.3 (Dako).
Single-cell PCR and Sequencing
TCR usage was analyzed by a single-cell RT-PCR protocol (21, 25). For TCR β-chain analysis, a panel of primers specific for all known TCR β-chain variable regions in combination with a β-chain constant region primer was used (26). RT-PCR amplicons were used as templates for a second round of PCR amplification using a panel of nested TCR β-chain-specific primers. PCR products were treated with Exonuclease I (NEB Biolabs) and shrimp alkaline phosphatase (Roche) and sequenced by the UNC Sequencing Core Facility. TCR sequence gene-usage identifications were performed using the SoDA software tool (27). Sequences were defined as identical if they shared the same Vβ and Jβ gene usage along with identical β-CDR3 regions.
Diversity Analysis
The Shannon entropy was chosen as the index of diversity because it is the only member of the family of valid diversity indices where the calculation derives equal weight from species richness (number of different clones recovered) and species frequency distribution (number of copies of each clone recovered); as such, the calculated diversity favors neither especially abundant nor especially rare species (as do the Simpson’s index and simple species richness, respectively) (28). This index has been used widely in ecology and has been used to quantify the diversity of hepatitis C viral quasispecies and MHC class II regulatory gene segments (28–30). The Shannon entropy of a T cell population is determined by two parameters: 1) the number of different T cell clones that are present, and 2) the frequency of each individual clone. Entropy is greatest when there are many different T cell clones and when there are few clones that are highly represented in the population (i.e. few “dominant” clones). In pooled samples, entropy increases with decreased sequence sharing between individual samples in the pool (i.e. low frequencies of shared or “public” clones). Intuitively, this index represents the intra-population variability of the potential interactions available to T cells in the pool. If S is the total number of unique clonotypes in the pool, and pi is the proportion of the pool represented by clonotype i, the Shannon entropy H is defined as:
In practice, the proportions pi are not known, however, and must be estimated from finite samples. Simple substitution of these estimates into the definition of H gives rise to sampling bias. The bias is itself estimable when the total number of unique clonotypes S in the sampled population is known (31). In the present case, S is not known. To address this problem, we have developed a Bayesian method to estimate the Shannon entropy accounting for clonotypes in the population that are unseen in the sample (Kepler, manuscript in preparation). Utilization of such a procedure is necessary because incomplete sampling could otherwise result in grossly underestimated entropy values and invalid comparisons between samples. Importantly, confidence intervals for the entropy estimation are also given by this method, which has been implemented in software and is available upon request.
Sequence Sharing Analysis
Sequences were defined as shared if they were present in samples taken from more than one mouse. Sequence sharing was calculated using a Python script.
Statistical Analyses
Data were analyzed using Prism 4.0 (GraphPad Software, San Diego, CA). Mann-Whitney U tests were done to evaluate population differences in percentage of clonotypes shared, number of tetramer-positive cells per islet, and percentage of CD8+ T cells that were tetramer-positive. The Kruskal-Wallis test with Dunn’s post-tests was used to evaluate population differences in TRBV 13-3 expression and graphical results displayed as dot plots with population mean indicated by horizontal bars. The Kaplan-Meier curve was used to determine the significance of the difference is diabetes incidence between treated and control mice. In all analyses, the significance level was 0.05.
T Cell Receptor Gene Nomenclature
Gene names are given according to the IMGT nomenclature (32), with older nomenclature occasionally included parenthetically for clarity. A conversion chart between the various nomenclatures is available at: http://imgt.cines.fr/textes/IMGTrepertoire/LocusGenes/#4 (33)
Results
TCRβ gene usage decreases in diversity over time in the islets, but not in the pancreatic lymph nodes and spleen of 8–14 week old NOD mice
Previous work from our lab and others have suggested that the T cell repertoire in the periphery and the islets of prediabetic NOD mice is overlapping (20, 21). This suggests that the CD8+ T cells are generated in the periphery and migrate to the islets where they function. Further, if the complexity of the response in the islets decreases- as would be expected for selection, then deletion of those clones would be more feasible, since they would have a more homogenous avidity. We have extended previous studies to examine the clones expressed in the periphery and islets at times before 20 weeks. By comparing three times we can examine the trajectory of the changes in the complexity of the T cell repertoire and therefore better predict the outcome of deletion.
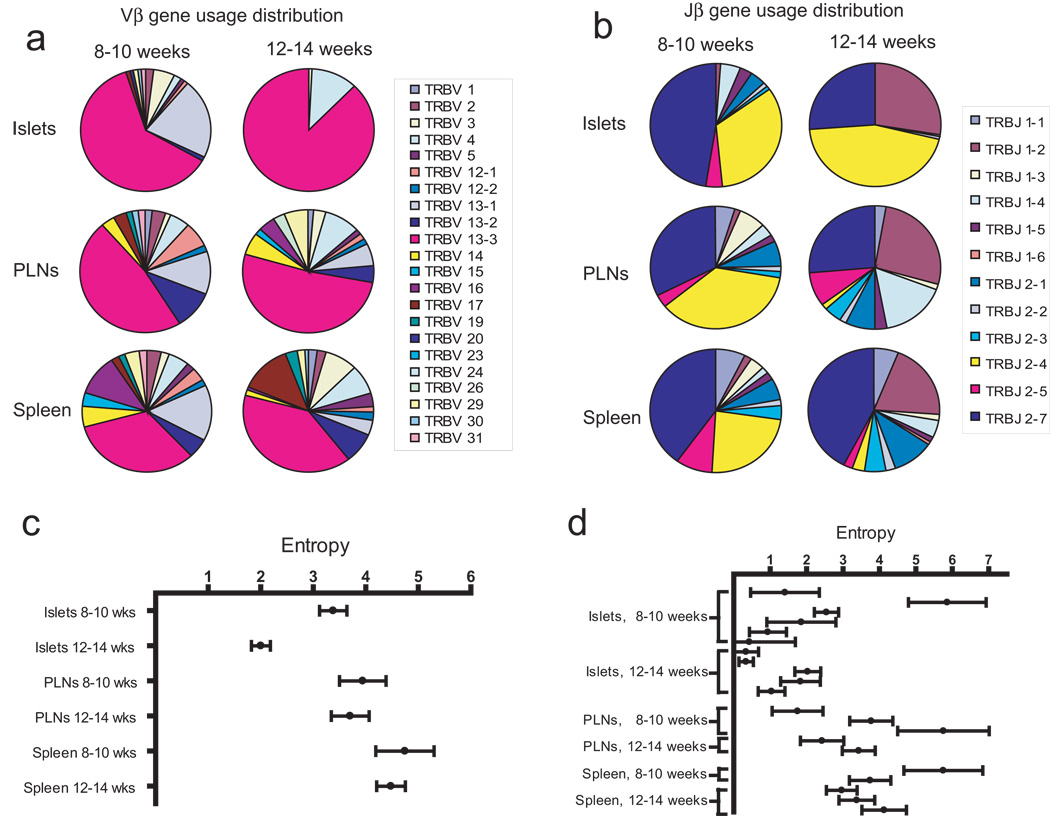
CD8+ NRP-V7+ T cells were sorted into individual wells and TCR usage determined for single cells. We began these experiments examining NRP-V7+ T cells because the authentic IGRP peptide was not available at the time, and many studies examining repertoire have already been done using NRP-V7+ T cells (34). We sequenced a total of 563 TCRβ chains from single cells. Results of these experiments are summarized in table I, and a list of these and other sequences recovered is presented in table S1. Vβ gene usage was highly restricted in the islets at 12–14 weeks of age (Fig. 1a). In all other tissues, Vβ usage was distributed among multiple Vβ families. TRBV 13-3 (old Vβ 8.1) was the dominant Vβ gene used in all tissues at all time points, and increased in dominance in the islets over time (Fig. 1a), characterized by an increasing portion of the pool that expressed TRBV 13-3 as well as a decreasing total number of Vβ genes represented. Jβ gene usage was also restricted in the islets at 12–14 weeks of age, with diversity in the islets at both ages less than that of the PLN and spleen. TRJB 2–4 and TRJB 2–7 were highly represented in all tissues at 8–10 weeks of age, with TRJB 2–7 continuing to be highly represented at 12–14 weeks of age in all tissues, in contrast the frequency of TRJB 2–4 decreased in the PLN and spleen but increased in the islets. TRJB 1–2 rose in frequency in all tissues over time and was a dominant Jβ gene in the islets at 12–14 weeks of age. These patterns of Vβ and Jβ usage are in agreement with prior work that showed dominance in the islets of TRBV 13-3 and TRJB 2–4 and 2–7 (12, 21, 25). The decrease in total Vβ and Jβ genes used in the islets indicates that the TCR repertoire becomes more restricted with age in NOD mice.
Table I. Summary of Sequence Data.
Summary of all TCRβ sequence data. NRP-V7-specific CD8+ T cells were single-cell sorted and their TCRβ genes amplified by RT-PCR and sequenced. Numbers of mice included, total sequences recovered, unique sequences recovered, and number of shared clones are listed for each group. A clonotype was defined as shared if it was recovered from more than one mouse. One mouse contributed more than one sample to this analysis (islets and PLNs taken from one 10 week old animal). Otherwise all mice contributed T cells derived from only one tissue.
| Sample | # mice | # sequences | # unique clonotypes |
# shared clonotypes |
|---|---|---|---|---|
| Islets 8–10 weeks | 6 | 97 | 34 | 7 (20%) |
| Islets 12–14 weeks | 5 | 148 | 14 | 10 (71%) |
| PLNs 8–10 weeks | 3 | 61 | 36 | 6 (17%) |
| PLNs 12–14 weeks | 2 | 68 | 34 | 8 (24%) |
| Spleen 8–10 weeks | 2 | 55 | 41 | 6 (15%) |
| Spleen 12–14 weeks | 3 | 134 | 72 | 6 (8%) |
Figure 1.
Diversity of NRP-V7+CD8+ T cells in NOD mice decreases over time in the pancreatic islets, but not in the pancreatic lymph nodes or spleen. NRP-V7-specific CD8+ T cells were single-cell sorted and their TCRβ genes amplified by RT-PCR and sequenced. (a) Vβ gene-usage distribution. (b) Jβ gene-usage distribution. (c) Entropy of pooled samples. Each data point represents the entropy of samples pooled by age of mice and tissue of origin. (d) Entropy of individual samples. Each data point represents cells derived from a single mouse. In c and d, Shannon entropy is used as an index of diversity, calculated using the estimateEntropy program, and reported with 95% confidence intervals represented by error bars.
While is it possible to examine these changes qualitatively, until recently quantitative assessment of changes in repertoire have been challenging. We have recently adapted methodology from ecology to combine both the number and diversity of TCRβ sequences using entropy calculations. Entropy calculation takes into account both the number of different TCRβ sequences and the population distribution among the different sequences, an improvement over simple counting of the number of different sequences. Entropy in the islets at 12–14 weeks was significantly less than at 8–10 weeks of age (Fig. 1c). This decrease was determined by both a declining overall number of unique clones and an increased frequency of the most abundant clones (table S1). In no other tissue was a significant difference seen between the two ages. In contrast to the pooled data, the non-pooled islet entropies showed no significant difference between the 8–10 and 12–14 week old samples, with the exception of a single 8–10 week mouse that had an entropy value of 5.86 (Fig. 1d). This sample had 13 sequences recovered that were all unique clones (Fig. S1), and this entropy value approaches maximal entropy. When this mouse is excluded from the 8–10 week-old pool, the pooled entropy is still significantly different between the 8–10 and 12–14 week-old pools. This implies that the frequency of shared clones between individual samples in the pools increases between 8–10 and 12–14 weeks of age, thus contributing to a decrease in the pooled entropy without a corresponding decrease in individual sample entropies over time. Sequence sharing increased over time in the islets, but not in the PLN and spleen.
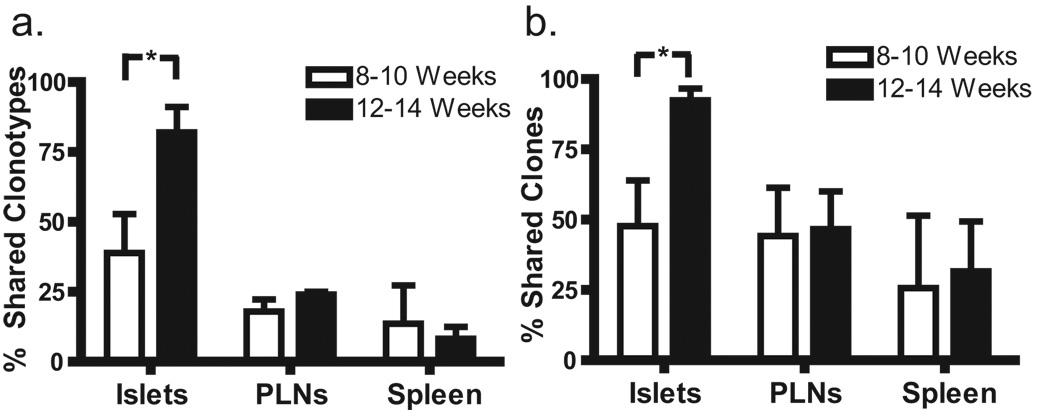
A major question remaining is: Do the repertoires in the periphery and the islets evolve independently or are they in equilibrium? In other words, are the effector T cells in the isets simply a sample of the periphery or do the two anatomic sites have different selective pressures. If increased sequence sharing occurs only in the islets, this implies the islets have different selective pressures. In order to confirm an increased number of public use clonotypes (i.e. clones shared among NOD mice) found in the islets of 12–14 versus 8–10 week-old animals contributed to the decrease in diversity over time, the sequence datasets were analyzed for the presence of shared clonotypes. Sequence sharing increased over time in the islets, but not in the PLN or spleen. This was the case regardless of whether unique clonotypes (Fig. 2a) or all T cell clones recovered (Fig. 2b) were considered. This suggests that the temporal decrease in pooled entropy seen in the islets was driven by increased sequence sharing, although the total number of unique clonotypes decreased and clonal dominance increased as well. The sequences of shared clonotypes and the samples from which they were recovered are reported in table S2. Fourteen out of eighteen shared clonotypes (78%) expressed TRBV 13-3, a markedly higher portion of clonotypes than at any age/tissue studied except for the 12–14 week-old islet sample (Fig. S1). Further, eight out of ten clonotypes recovered from more than two mice expressed TRBV 13-3. Of note, the most frequently shared clone (TRBV 13-3 TRJB 2–4 ASSDSQNTLY) differs from the pathogenic clone NY8.3 by only one amino acid, and three out of eighteen shared clones used the CDR3 motif ASSDXXNTLY.
Figure 2.
Sequence sharing among NOD mice increases in the islets over time, but not in the PLN or spleen. A sequence was defined as shared if it was recovered from more than one mouse, and the proportion of shared sequences in each group is shown. (a) Sharing of unique clonotypes. The frequency of shared clonotypes in the 12–14 week islet group was greater than in the 8–10 week islet group. n = 6 mice in the 8–10 week islet group and 5 mice in the 12–14 week islet group. * P = 0.026, One-tailed Mann-Whitney. (b) Sharing of all T cell clones recovered. Again, the frequency of shared sequences was greater in the 12–14 week islet group. n = 6 mice in the 8–10 week islet group and 5 mice in the 12–14 week islet group. * P = 0.041, One-tailed Mann-Whitney.
Based on the result that pooled diversity decreased while sequence sharing increased in the islets but not other tissues, we reasoned that clonal selection was occurring selectively in the islet pool. The increased prevalence in all tissues and increasing dominance in the islets of the TRBV 13-3 bearing clones, along with the high frequency of TRBV 13-3 expression in the public clones, imply that the TRBV 13-3 bearing clones are selected. The presence of this defined public subset of dominant clones further suggests TCRβ repertoire status may be important in progression of islet autoimmunity.
Together this data shows that TCR diversity decreases over time and is consistent with either strong founder effects or selection in the islets, rather than equilibrium with the periphery antigen specific pool.
Kd-NRP-V7-SAP tetramers deplete epitope-specific T cells in vitro and in vivo
The sequence analysis we presented suggests that the populations are relatively homogenous, and combined with data from Armani (34) suggests that elimination of high affinity clones might be effective in preventing progression of diabetes. Further the high prevalence of NRP-V7+ in the islets (22, 35) suggests that they are critical in the destruction of β cells and so would be good targets for removal.
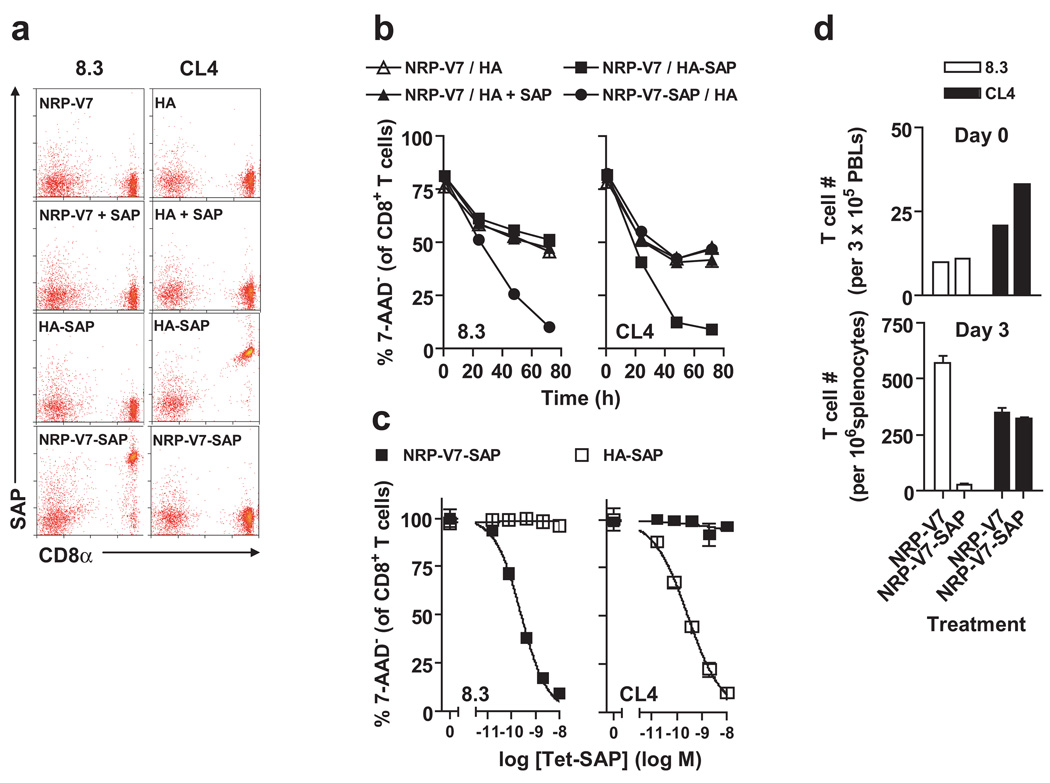
Our previous work using toxin-coupled tetramers showed that in model systems LCMV gp33 specific P14 transgenic T cells were effectively deleted both in vitro and following adoptive transfer in vivo without significant toxicity to the mice (17). We hypothesized that treating NOD mice with Kd-NRP-V7-SAP tetramers would alter the TCR repertoire of this diabetogenic population and potentially delay the onset of T1D in treated mice. We tested the ability of similar toxin coupled tetramers assembled with NRP-V7 to remove 8.3 TCR transgenic CD8+ T cells. We cultured CD8+ T cells from the NOD 8.3 TCR transgenic mouse (which bears transgenic CD8+ T cells that react to H2Kd-NRP-V7) and the NOD CL4 TCR transgenic mouse (which bears transgenic CD8+ T cells that react to the influenza peptide HA presented by H2Kd) with cognate and non-cognate toxic tetramers. Binding and cytotoxicity were specific for cognate tetramer relative to non-cognate tetramer and free saporin (Fig. 3). Kd-NRP-V7-SAP selectively depleted the 8.3 cells with very little toxicity to a non-targeted population (Fig. 4a).
Figure 3.
Validation of specific binding and killing of CD8+ T cells by SAP-coupled Kd tetramers in vitro and in vivo. (a) Kd tetramers prepared with SA-SAP retain binding specificity, and free SAP does not bind CD8+ T cells. Peripheral blood lymphocytes from NOD 8.3 and NOD CL4 mice were incubated with tetramers at 4°C for 1 h, washed extensively, and probed for surface SAP binding with polyclonal anti-SAP Abs. (b) T cells are killed by cognate SAP-coupled tetramers in 72 h. Cultured T cells were harvested at 1, 24, 48 or 72 h. (c) T cells are killed by cognate SAP-coupled tetramers in dose-dependent fashion. T cells were harvested at 72 h; results are normalized to percent survival with non-toxic tetramer treatment alone. The EC50 values for killing of NOD 8.3 T cells were 0.254 and 0.564 nM in two independent experiments. In (b) and (c), T cells were incubated with tetramers at 37°C for 1 h, then washed and cultured in medium alone for the indicated times; harvested cells were analyzed by flow cytometry after staining with anti-CD8 mAb and 7-AAD. (d) The Kd-NRP-V7-SAP tetramer can eliminate naïve cognate CD8+ T cells in vivo. Purified Thy1.2+ NOD 8.3 and NOD CL4 T cells (1 × 106 each) were mixed and transferred i.v. into NOD Thy1.1+ hosts. To verify equivalent transfer, PBLs were collected from recipient mice 1 day later and analyzed for Thy1.2+ tetramer+ CD8+ T cells (upper panel); mice were then injected i.v. with either Kd-NRP-V7 or Kd-NRP-V7-SAP tetramers. Three days after treatment, splenocytes were stained with Kd-HA-PE, Kd-NRP-V7-AlexaFluor647, 7-AAD, and an anti-CD8 mAb, and analyzed by flow cytometry, showing the loss of NOD 8.3, but not NOD CL4 control, T cells following treatment with the Kd-NRP-V7-SAP tetramer (lower panel).
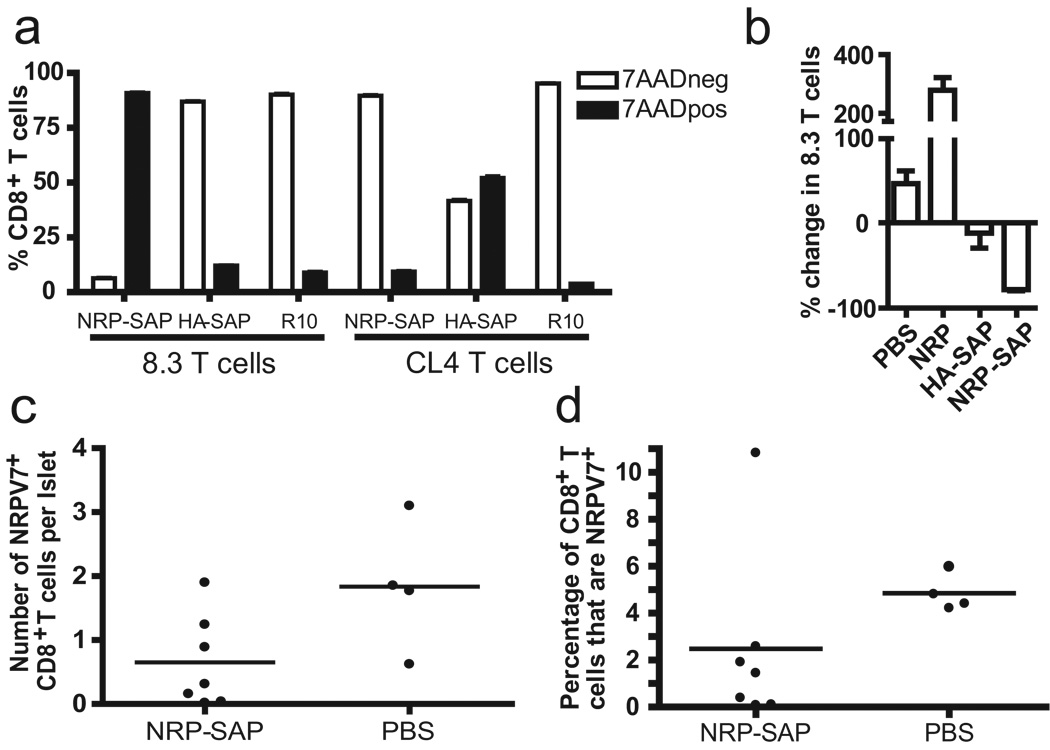
Figure 4.
Toxic tetramers selectively deplete epitope-specific T cells in vitro and in vivo. (a) NOD 8.3 or CL4 splenocytes were cultured 3 days in RPMI containing 10% FCS with cognate or irrelevant toxic tetramer or without toxic tetramer, then stained with anti-CD8 and 7AAD to measure viability. NRP-SAP refers to NRP-V7-SAP. Percent of tetramer-positive CD8+ T cells is shown. (b) Kd-NRP-V7-SAP tetramer eliminates activated diabetogenic CD8+ T cells in vivo. Purified NOD-8.3 T cells (Thy1.2+) and CD8-depleted NOD splenocytes (Thy1.1+) were co-transferred into NOD scid hosts, followed by a priming injection of IGRP peptide. Five days later, PBLs were collected to verify equivalent transfer. NOD scid mice were then injected i.v. with the indicated tetramer (22 pmol) or PBS. Seven days after treatment, PBLs were analyzed by flow cytometry, showing loss of NOD-8.3 T cells in mice that received the Kd-NRP-V7-SAP tetramer. Results are expressed as the mean percentage change vs. treatment day 0. (c) NRP-V7+CD8+ T cells are reduced in NOD mice treated with Kd-NRP-V7-SAP. CD8+ cells were isolated from the islets of Kd-NRP-V7-SAP treated and control mice and analyzed by flow cytometry. One mouse from each group had less than 2 CD8+ T cells per islet (less than 5% of the average number) and only one tetramer-positive T cell in the sample. These NOD mice were considered to have not developed insulitis and were excluded from this analysis. Total number of tetramer-positive CD8+ T cells per islet is shown. P = 0.054, One-tailed Mann-Whitney. (d) Proportion CD8+ T cells that were tetramer-positive is shown. P = 0.036, One-tailed Mann-Whitney.
We then evaluated the ability of Kd-NRP-V7-SAP to selectively eliminate naïve NOD.8.3 T cells in vivo (Fig. 4b). Purified 8.3 CD8+ T cells mixed with CD8-depleted NOD splenocyte helpers were transferred into NOD scid recipients, which in turn were treated with native IGRP206–214 peptide. Five days later, NOD scid mice received a single injection of PBS, NRP-V7, Kd-NRP-V7-SAP, or Kd-HA-SAP tetramers. After seven days, 8.3 CD8+ T cells had modestly expanded in PBS-treated mice; as expected, this expansion was greatly enhanced by exposure to non-toxic cognate tetramer (36, 37). In contrast, treatment with Kd-NRP-V7-SAP decreased 8.3 CD8+ T cells in the blood by >75%, similar to the depletion noted in the spleens of lymphoreplete mice, showing that activated diabetogenic transgenic CD8+ T cells are depleted by cytotoxic tetramer. Thus these experiments demonstrate diabetogenic T cells can be removed in vivo.
Kd-NRP-V7-SAP treatment decreases both the absolute number and frequency of NRP-V7+ T cells in the islet-infiltrating CD8+ T cell pool
We then assessed our ability to remove a heterogeneous pool of NRP-V7+ CD8+ T cell clonotypes from NOD mice as a preamble to testing the efficacy of direct killing of diabetogenic T cells to block progression to T1D. To test this, 13 NOD female mice were treated with Kd-NRP-V7-SAP or PBS. Islet-infiltrating NRP-V7+CD8+ T cells were isolated from treated and untreated mice at 3 weeks post treatment (11 weeks of age) and analyzed by flow cytometry. Both the absolute number per islet and proportion of NRP-V7+ CD8+ T cells decreased with Kd-NRP-V7-SAP treatment (Fig. 4c–d). Fifty percent of the treated mice showed near complete depletion of NRP-V7+ CD8+ T cells, while the other half had substantial tetramer-positive T cells remaining, although nearly all were present at a lower frequency than the PBS control (P<.02 Fisher exact test).
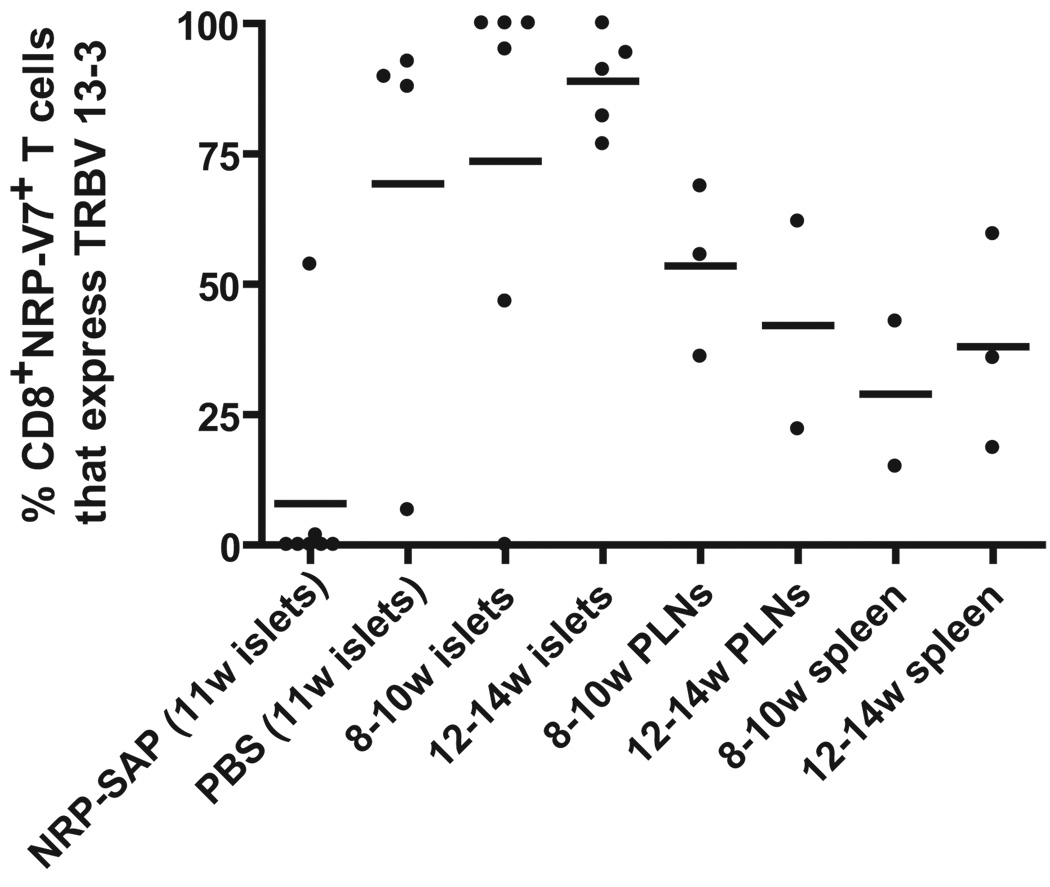
NRP-V7+CD8+ T cells that express TRBV 13-3 concentrate in the islets and are selectively depleted by Kd-NRP-V7-SAP treatment
It is possible that the depletion we observed was either stochastic or selected. If stochastic, we would expect the IGRP tetramer+ T cells would be drawn from the same distribution of TCRβ genes before and after treatment. If the cells were selected by strength of tetramer binding, we expect to see a selective depletion of some classes. TRBV 13-3 bearing clones were present at a high frequency in all tissues at all times and increased in dominance and sharing in the islets in unmanipulated NOD mice. In order to determine the effect of Kd-NRP-V7-SAP treatment on TRBV 13-3 frequency in the NRP-V7+CD8+ T cell pool, TCRβ sequences were analyzed for frequency of TRBV 13-3 expression (Fig. 5). As indicated in Figure 1, TRBV 13-3 expression was more prevalent in the NRP-V7+CD8+ T cell clones derived from the islets than in those from the PLN or spleen. At 11 weeks in the PBS-injected NOD mice and 12–14 weeks in untreated NOD mice, almost all of the tetramer-positive clones expressed TRBV 13-3. In the 8–10 week-old islet pool, 4/6 NOD mice showed the same exquisite dominance of TRBV 13-3. In NOD mice receiving Kd-NRP-V7-SAP, 5 mice showed complete absence of TRBV 13-3 expressing clones, one mouse had 1/52 (1.9%) clones express TRBV 13-3, and another had 21/39 (53.8%) express TRBV 13-3. In the islets of all but one treated mouse, the percentage of NRP-V7-specific CD8+ T cells that used TRBV 13-3 was less than in the spleen at either time point. These results demonstrate that TRBV 13-3 bearing NRP-V7+ CD8+ T cells are largely found in the islets and are depleted by Kd-NRP-V7-SAP treatment. Thus the depletion by tetramer is not random, but selective.
Figure 5.
NRP-V7+CD8+ T cells that express TRBV 13-3 accumulate in the islets and are deleted by Kd-NRP-V7-SAP. T cells were isolated from the islets of NOD mice 3 weeks post-initial treatment with Kd-NRP-V7-SAP, 3 weeks post-treatment with PBS, or from the islets, PLN, and spleen of unmanipulated NOD mice 8–14 weeks of age. Each data point represents proportion of islet-infiltrating NRP-V7+CD8+ T cells from a single mouse that expressed TRBV 13-3. One mouse from the Kd-NRP-V7-SAP treated group and one mouse from the PBS group had less than 2 CD8+ T cells per islet (less than 5% of the average number) and only one tetramer-positive T cell in the sample. These mice were considered to have not developed insulitis and were excluded from this analysis. Data were analyzed using the Kruskal-Wallis test with Dunn’s post-tests. Significant differences were found between the Kd-NRP-V7-SAP and PBS treated NOD mice (P < 0.05), 8–10 week islets (P < 0.01), and 12–14 week islets (P < 0.001).
Toxin coupled tetramers produced long-term depletion of tetramer-specific CD8+ T cells in the islets
A critical question for the use of depleting agents is how long the effects of treatment persist. Treatment with depleting antibodies such as anti CD3 is transient in humans (38) and nearly absent in NOD mice (39). Although the mechanism of action of anti-CD3 therapy is not clear, it is unlikely to function by lymphocyte depletion alone (15). Treatment with toxic tetramer on the other hand does seem likely to function by removal of reactive cells.
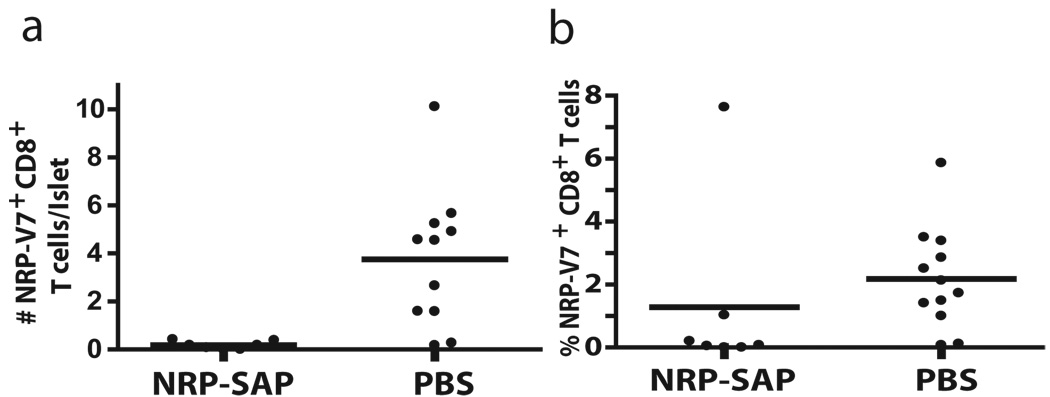
To test whether altering the repertoire of pathogenic NRP-V7+CD8+ T cells would affect T1D, 10 mice per group were injected with toxic tetramer or PBS and monitored for development of diabetes up to 54 weeks of age. Islet infiltrating NRP-V7+ CD8+ T cells were isolated upon development of overt diabetes (2 blood sugar readings over 250 mg/dl) or at 54 weeks of age. NOD mice treated with Kd-NRP-V7-SAP had significantly lower numbers of islet infiltrating NRP-V7+ CD8+ T cells as measured by either the number of NRP-V7+ CD8+ T cells per islet (p=0.002), or the percent of NRP-V7+ cells in the CD8+ T cell population (p=0.021) even up to 44 weeks after the last treatment with Kd-NRP-V7-SAP (Fig. 6). The reduction in number of NRP-V7+ CD8+ T cells was not due to a reduction in overall numbers of CD8+ T cells at the time of measurement, since both Kd-NRP-V7-SAP and PBS treated mice had similar numbers of CD8+ T cells per islet (data not shown). In addition, the mean fluorescence intensity of the NRP-V7+ CD8+ T cells left in the islets of the Kd-NRP-V7-SAP treated NOD mice was also lower, 151 +/− 103 versus 372 +/− 242 for the PBS treated NOD mice.
Figure 6.
Kd-NRP-SAP caused long-term depletion of tetramer-specific CD8+ T cells in the islets. Female NOD mice were given 3 i.v. injections of Kd -NRP-SAP or PBS over 2 weeks beginning at 8 weeks of age. At onset of diabetes or 54 weeks, NRP+ CD8+ CD3+ T cells were isolated from the islets and analyzed by flow cytometry. Absolute number of tetramer-positive CD8+ T cells per islet (a) and proportion of CD8+ T cells that were tetramer positive (b) are shown. PBS treatment group includes female NOD mice from 2 different toxic tetramer trials. ** P = 0.002, One-tailed Mann-Whitney. * P = 0.021, One-tailed Mann-Whitney.
Treatment with toxin-coupled tetramers can specifically delay the onset of T1D
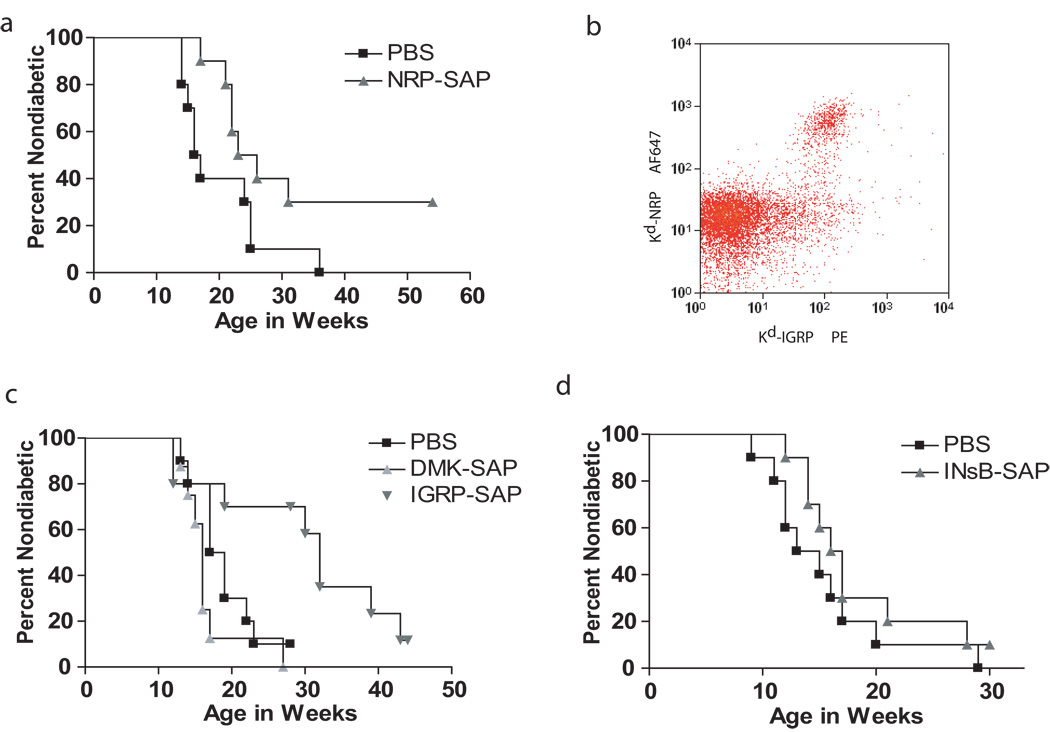
Notably, the onset of overt diabetes was significantly delayed in NOD mice treated with Kd-NRP-V7-SAP versus the control group (24.5 and 16.5 weeks of age respectively, p=0.04) (Fig. 7a). Thus treatment with toxic tetramer resulted in the long-term depletion of NRP-V7+ CD8+ T cells in the islets, as well as significantly delayed the onset of overt diabetes in vivo. This result is consistent with the observation that NRP-V7+ CD8+ T cells are able to induce T1D and are important effector cells in disease progression.
Figure 7.
Kd-NRP-V7-SAP and Kd-IGRP-SAP delayed onset of diabetes but not Kd-INsB-SAP or Dd-DMK-SAP. (a,c,d) Ten NOD mice per group were given 3 i.v. injections of the indicated toxic tetramer over 2 weeks beginning at 8 weeks of age. A Kaplan-Meier curve is shown above with diabetes incidence as the dependent variable. (a) Kd-NRP-SAP treatment significantly reduced diabetes incidence (logrank test, P = 0.04). Mean time to onset, PBS = 16.5 weeks, Kd-NRP-SAP = 24.5 weeks (b) NRP-V7-specific CD8+ T cells are a subset of the IGRP-specific CD8+T cells. Lymphocytes were isolated from the islets of a NOD mouse and stained with anti-CD8 PerCp, anti-CD3 AF488 and anti-CD19 Pacific Blue antibodies in addition to two tetramers, Kd-NRP-V7-SA-AF647 and Kd-IGRP-SA-PE. Cells were analyzed by flow cytometry. The CD8+CD3+T cells are shown in the dot plot. (c) Kd-IGRP-SAP but not Kd-DMK-SAP significantly delayed onset of diabetes (logrank test, P = 0 .02). Mean time to onset, PBS = 18 weeks, Kd-IGRP-SAP = 32 weeks, and Dd-DMK-SAP = 16 weeks (d) Kd-INsB-SAP did not reduce diabetes incidence. Mean time to onset, PBS = 14 weeks, Kd-INsB-SAP = 16.5 weeks. Blood ALT( alanine aminotransferase) levels (indicative of liver damage) measured after each dose of toxic tetramer did not rise above 350, consistent with mild transient effects.
Treatment with NRP-V7-SAP did not completely block progression to T1D and we wondered whether there were other IGRP+ CD8+ T cells that were not eliminated with NRP-V7-SAP tetramers. NRP-V7 was originally identified as a mimotope that would activate 8.3 T cells both in vitro and in vivo (40). More recently IGRP has been described as the natural ligand of 8.3 cells. We therefore examined the similarity of staining patterns of Kd-IGRP and -NRP-V7 tetramers on NOD CD8+ cells isolated from the islets (Fig. 7b). We found that while all of the Kd-NRP-V7 staining CD8+ T cells also bound Kd-IGRP, a substantial population of Kd-IGRP+ CD8+ T cells was Kd-NRP-V7 negative. This demonstrates that there are potentially diabetogenic CD8+ T cells specific to IGRP that will not be removed by NRP-V7-SAP. Alternatively one could reason that it is better to deplete this high affinity diabetogenic IGRP+ population and leave the low affinity IGRP cells expand to fill the space previously occupied by the high affinity T cells since depletion of all of the IGRP+ CD8+ T cells would allow other high affinity diabetogenic cell of other epitopes to fill in the gap (10, 12).
We then tested the ability of Kd-IGRP tetramers to delay onset of T1D, since we thought that they might be more effective that Kd-NRP-V7-SAP. Treatment with Kd-IGRP-SAP was found to be more effective than Kd-NRP-V7-SAP (Fig. 7c). Indeed 50% of the IGRP treated mice did not develop diabetes after 30 weeks compared to 100% of the controls. Mean onset of diabetes was 32 weeks for Kd-IGRP-SAP compared to 24.5 weeks for Kd-NRP-V7-SAP treated animals and 16.5 weeks with PBS. Thus although treatment with Kd-IGRP-SAP was more efficacious than Kd-NRP-V7 when begun at 8 weeks, it did not completely prevent the progression to T1D
Several other class I epitopes have been described in NOD mice (41). These include peptides from DMK and InsB chain. InsB-G9V was used to increase the MHCI stability with minimal effect on TCR-MHCI interaction (42). Since these epitopes also likely contribute to the pathogenesis of diabetes, we chose to examine the impact of deleting these CD8+ T cells. We prepared saporin coupled tetramers loaded with each of these peptides and compared their ability to delay the development of T1D in NOD mice. We compared these treatments with tetramers complexed with NRP-V7 and IGRP peptides. Neither Kd-InsB nor Db-DMK tetramers were able to delay the onset of T1D. This was surprising given that that NOD mice genetically tolerant to Ins-B do not progress to T1D (43). The age of treatment was not a major factor since a similar result was obtained when NOD mice were treated at 4 weeks of age with the Kd-InsB-SAP (data not shown). This result also demonstrates the specificity of deletion. It shows that toxin coupled tetramer alone, irrespective of specificity is not sufficient to delay the onset of T1D.
Discussion
In this paper we show that toxin coupled tetramers significantly alter the T cell repertoire of NOD mice. The TCRβ repertoire of Kd-NRP-V7+ CD8+ T cells in NOD mice is known to be highly restricted at 20 weeks of age and to preferentially use TRBV 13-3 and TRJB 2–4 or 2–7 (21). Our study represents the first dissection of the TCRβ repertoire in younger NOD mice. In agreement with earlier observations, we found dominance of TRBV 13-3 and TRBJ 2–4/2–7 to be true for 8–14 week-old NOD mice as well. We show here for the first time that diversity of the Kd-NRP-V7+CD8+ T cell pool decreases in the islets over time but not in the PLN or spleen. Though this decrease in diversity is characterized by a declining number of unique clonotypes and an increase in frequency of the dominant clones, the primary driver is an increase in sequence sharing among clones present in the islets at 12–14 weeks of age. This suggests a progressive selective pressure in the islets which is conserved among NOD mice. It also implies that selection of NRP-V7-specific CD8+ T cell clones over time is not uniform in all tissue compartments. Rather, selection in the islets is more robust for this T cell pool.
We further show that a dominant subset of NRP-V7+CD8+ T cell clones, those expressing TRBV 13-3, is enriched and increasingly public in the islets over time. Shared clonotypes were more likely than non-shared clonotypes to use TRBV 13-3, which suggests that the predominance of these clones in the shared pool is not primarily a consequence of their increased overall frequency in the total pool. Further, the TRBV 13-3+ NRP-V7+CD8+ T cell population was selectively depleted from the NRP-V7+CD8+ T cell pool in the islets by treatment with Kd-NRP-V7 tetramer conjugated to the ribosomal toxin saporin. It is intriguing that Kd-NRP-V7-SAP mediated depletion of epitope-specific CD8+ T cells was incomplete two weeks post-treatment while the depletion of Kd-NRP-V7+ CD8+ T cells measured up to 44 weeks later was more complete (Figure 4c, Figure 6a). Prior work by our group has shown that CD8+ T cells expressing TCRs exhibiting decreased binding avidity for toxic tetramer are relatively resistant to toxic-tetramer mediated depletion (17). Thus we demonstrated a potential for a therapeutic benefit where deletion of a single specificity can alter the outcome of disease progression.
Peptide-MHC tetramers assembled with the saporin are promising agents for direct epitope-specific depletion of T cells. This study presents the first evidence of a beneficial effect of toxic tetramer administration. Toxic tetramer assembled with IGRP was nearly as effective at decreasing diabetes incidence as low-affinity peptides in earlier studies (12, 20). In addition, peptide treatment was only effective when initiated at 4 weeks of age and disease progression was unaltered when peptide treatment was initiated in 10 week-old NOD female mice. Here, however, a marked effect on T1D was detected when 8 week-old NOD female mice were treated with toxic tetramer, when islet infiltrates are well established. Further, in earlier experiments continued peptide injections were necessary while in our experiments, NOD mice were treated only 3 times over a 10 day period. We believe this technology represents a new strategy for in vivo immunomodulation in contexts where repeated administration of peptide may be undesirable or where a stable platform for direct rather than APC-mediated depletion is needed.
It is not surprising that toxic tetramer treatment eliminated NRP-V7 specific CD8+ T cells from the islets and slowed the progression of diabetes, but most NOD mice still progressed to diabetes without NRP-V7+ CD8+ T cells. This adds more evidence that there are multiple driving epitopes in β cell autoimmunity. Others have depleted or tolerized NRP-V7 reactive CD8+ T cells in NOD mice without causing delay of T1D (10, 12). This is consistent with human data that shows the preproinsulin epitope in many but not all A2+ patients arises before other detectable epitopes (44).
Interestingly tolerization to proinsulin 2 prevents both T1D and development of IGRP reactive CD8+ T cells (10) and mice transgenic for an insulin gene that produces an altered insulin B chain, also do not develop TID (11). This suggests that the response to proinsulin and insulin epitopes are crucial as direct effectors or a critical check point in epitope spreading and precede IGRP directed autoimmunity. We were surprised to find that the progression of diabetes was unaffected when 4 week old NOD mice with treated with Kd-InsB-SAP (data not shown). There are several explanations for our results. We might not have sufficiently depleted the InsB-specific CD8+ T cells, allowing them to play a critical role in initiating the autoimmune response. Alternatively, depletion at 4 weeks may still be too late; if progression to TID would already have passed the InsB check point, there would be no effect. On the other hand, proinsulin or preproinsulin CD8+ T cell epitopes may be better candidates for intervention. Finally, our study does not address the role of the CD4 response to insulin which is known to be diabetogenic (43). The inclusion of toxin coupled class II tetramers with specificity for diabetogenic CD4+ T cells might well enhance the efficacy of treatment.
The magnitude of protection in our study (30%) was similar to that offered by low affinity NRP-I4 peptide (12). One possible implication of this is that Kd-NRP-V7-SAP tetramer treatment depletes the same population of “high-avidity” clones that are depleted by peptide. Mean fluorescence intensity values for islet-infiltrating tetramer-positive T cells were significantly lower in the treated mice. This supports the idea that non-depleted clonotypes, presumably of lower avidity, expand and in turn affect disease progression. In light of this, it will be interesting to examine depletion efficacy, repertoire changes, and decrease in diabetes incidence after treatment with toxic tetramers assembled with IGRP and other altered peptide ligands of this peptide, as NRP-V7 is not be the optimal choice for depletion of diabetogenic T cells. The treatment with IGRP deletes both high and moderate affinity clones, resulting in better protection. Our data show that IGRP assembled toxin-tetramers are superior to NRP-V7. Somewhat surprisingly, we saw no ability of tetramers assembled with either InsB peptide, or with DMK peptide to prolong the disease free interval. This might not be surprising since the frequency of both Kd-InsB and Db-DMK specific CD8+ T cells are low, even in untreated NOD mice. This result also suggests that the best targets are clonotypes found at a high frequency, which in turn likely reflects a key role in driving β cell autoimmunity. It further argues that if epitope spreading is a critical event in pathogenesis, both DMK and InsB are dependent on IGRP, although data from others would argue the reverse (11). Alternatively, the responses to these three epitopes might be independent of each other and required to interact in a complex way to produce diabetes. Regardless, peptide-MHC tetramers assembled with the saporin are promising agents for direct epitope-specific depletion of T cells.
Toxin coupled tetramers represent a new strategy for in vivo immunomodulation. We show epitope specific depletion of CD8+ T cells using saporin coupled MHC Class I tetramers. This approach is advantageous over current approaches such as tolerization with peptide or use of T cell-depleting antibodies since peptide treatments can cause proliferation of T cells and deletion of a broad swath of T cells, leaving the risk of broad immunosuppression. In addition, this study offers a first look into the clonotype dynamics in young, pre-diabetic NOD mice and examines the increasingly public islet infiltrating clonotypes with IGRP specificity. Finally we demonstrate that toxic tetramer depletion of IGRP-reactive CD8+ T cells is long term and beneficial in delaying TID.
Supplementary Material
Acknowledgements
We thank Joan Kalnitsky and Larry Arnold for help with flow cytometry and single-cell sorting. We also thank Garrick Talmage, Shaun Steele and Cindy Hensley for help with mice and experiments.
Footnotes
J.F. and P.H. were funded by a grant from the Juvenile Diabetes Research Foundation (1-2008-24). J.F. was funded by a grant from the National Institutes of Health (A1052435). P.H. was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases / National Institutes of Health (K08-DK082264).
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Abbreviations: Type I Diabetes, TID; pancreatic lymph node, PLN; islet-specific glucose-6-phosphatase catalytic subunit-related protein, IGRP; Insulin B, InsB; dystophia myotonica kinase, DMK; streptavidin, SA; saporin, SAP
Supplementary Figure 1. Percentage of TRBV 13-3 NRP-V7+CD8+ cell T clonotypes. TRBV 13-3 usage is highest for the shared clonotypes and the 12–14 week islet pools. For each tissue/age pool and for the pool of shared clonotypes, the percentage of clonotypes that expressed TRBV 13-3 is shown.
References
- 1.Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmun Rev. 2008;7:550–557. doi: 10.1016/j.autrev.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 3.Rainbow DB, Esposito L, Howlett SK, Hunter KM, Todd JA, Peterson LB, Wicker LS. Commonality in the genetic control of Type 1 diabetes in humans and NOD mice: variants of genes in the IL-2 pathway are associated with autoimmune diabetes in both species. Biochem Soc Trans. 2008;36:312–315. doi: 10.1042/BST0360312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the development of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 5.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 6.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 7.Wong FS, Janeway CA., Jr The role of CD4 vs. CD8 T cells in IDDM. J Autoimmun. 1999;13:290–295. doi: 10.1006/jaut.1999.0322. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, Santamaria P, DiLorenzo TP. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamurthy B, Mariana L, Gellert SA, Colman PG, Harrison LC, Lew AM, Santamaria P, Thomas HE, Kay TW. Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. J Immunol. 2008;180:4458–4464. doi: 10.4049/jimmunol.180.7.4458. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, Colman PG, Harrison LC, Lew AM, Thomas HE, Kay TW. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest. 2006;116:3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama M, Beilke JN, Jasinski JM, Kobayashi M, Miao D, Li M, Coulombe MG, Liu E, Elliott JF, Gill RG, Eisenbarth GS. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest. 2007;117:1835–1843. doi: 10.1172/JCI31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han B, Serra P, Amrani A, Yamanouchi J, Maree AF, Edelstein-Keshet L, Santamaria P. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med. 2005;11:645–652. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- 13.Chaillous L, Lefevre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, Bouhanick B, Mogenet A, Nicolino M, Carel JC, Lecomte P, Marechaud R, Bougneres P, Charbonnel B, Sai P. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet. 2000;356:545–549. doi: 10.1016/s0140-6736(00)02579-4. [DOI] [PubMed] [Google Scholar]
- 14.Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crino A, Spera S, Suraci C, Multari G, Cervoni M, Manca Bitti ML, Matteoli MC, Marietti G, Ferrazzoli F, Cassone Faldetta MR, Giordano C, Sbriglia M, Sarugeri E, Ghirlanda G. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia. 2000;43:1000–1004. doi: 10.1007/s001250051482. [DOI] [PubMed] [Google Scholar]
- 15.Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, Sayre P, Bianchine P, Wong E, Seyfert-Margolis V, Bourcier K, Bluestone JA. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132:166–173. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 17.Hess PR, Barnes C, Woolard MD, Johnson MD, Cullen JM, Collins EJ, Frelinger JA. Selective deletion of antigen-specific CD8+ T cells by MHC class I tetramers coupled to the type I ribosome-inactivating protein saporin. Blood. 2007;109:3300–3307. doi: 10.1182/blood-2006-06-028001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penaloza-MacMaster P, Masopust D, Ahmed R. T-cell reconstitution without T-cell immunopathology in two models of T-cell-mediated tissue destruction. Immunology. 2009;128:164–171. doi: 10.1111/j.1365-2567.2009.03080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan RR, Wong P, McDevitt MR, Doubrovina E, Leiner I, Bornmann W, O'Reilly R, Pamer EG, Scheinberg DA. Targeted deletion of T-cell clones using alpha-emitting suicide MHC tetramers. Blood. 2004;104:2397–2402. doi: 10.1182/blood-2004-01-0324. [DOI] [PubMed] [Google Scholar]
- 20.Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature. 2000;406:739–742. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- 21.Wong CP, Stevens R, Long B, Li L, Wang Y, Wallet MA, Goudy KS, Frelinger JA, Tisch R. Identical beta cell-specific CD8(+) T cell clonotypes typically reside in both peripheral blood lymphocyte and pancreatic islets. J Immunol. 2007;178:1388–1395. doi: 10.4049/jimmunol.178.3.1388. [DOI] [PubMed] [Google Scholar]
- 22.Young E, Hess P, Arnold L, Tisch R, Frelinger J. Islet Lymphocyte Subsets in Male and Female NOD mice are Qualitatively Similar but Quantitatively Distinct. Autoimmunity. 2009;42:678–691. doi: 10.3109/08916930903213993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poligone B, Weaver DJ, Jr, Sen P, Baldwin AS, Jr, Tisch R. Elevated NF-kappaB activation in nonobese diabetic mouse dendritic cells results in enhanced APC function. J Immunol. 2002;168:188–196. doi: 10.4049/jimmunol.168.1.188. [DOI] [PubMed] [Google Scholar]
- 24.Skowera A, Ellis RJ, Varela-Calvino R, Arif S, Huang GC, Van-Krinks C, Zaremba A, Rackham C, Allen JS, Tree TI, Zhao M, Dayan CM, Sewell AK, Unger W, Drijfhout JW, Ossendorp F, Roep BO, Peakman M. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong CP, Li L, Frelinger JA, Tisch R. Early autoimmune destruction of islet grafts is associated with a restricted repertoire of IGRP-specific CD8+ T cells in diabetic nonobese diabetic mice. J Immunol. 2006;176:1637–1644. doi: 10.4049/jimmunol.176.3.1637. [DOI] [PubMed] [Google Scholar]
- 26.Baker FJ, Lee M, Chien YH, Davis MM. Restricted islet-cell reactive T cell repertoire of early pancreatic islet infiltrates in NOD mice. Proc Natl Acad Sci U S A. 2002;99:9374–9379. doi: 10.1073/pnas.142284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volpe JM, Cowell LG, Kepler TB. SoDA: implementation of a 3D alignment algorithm for inference of antigen receptor recombinations. Bioinformatics. 2006;22:438–444. doi: 10.1093/bioinformatics/btk004. [DOI] [PubMed] [Google Scholar]
- 28.Jost L. Partitioning diversity into independent alpha and beta components. Ecology. 2007;88:2427–2439. doi: 10.1890/06-1736.1. [DOI] [PubMed] [Google Scholar]
- 29.Cowell LG, Kepler TB, Janitz M, Lauster R, Mitchison NA. The distribution of variation in regulatory gene segments, as present in MHC class II promoters. Genome Res. 1998;8:124–134. doi: 10.1101/gr.8.2.124. [DOI] [PubMed] [Google Scholar]
- 30.Arenas JI, Gallegos-Orozco JF, Laskus T, Wilkinson J, Khatib A, Fasola C, Adair D, Radkowski M, Kibler KV, Nowicki M, Douglas D, Williams J, Netto G, Mulligan D, Klintmalm G, Rakela J, Vargas HE. Hepatitis C virus quasi-species dynamics predict progression of fibrosis after liver transplantation. J Infect Dis. 2004;189:2037–2046. doi: 10.1086/386338. [DOI] [PubMed] [Google Scholar]
- 31.Chao A, Shen T-J. Nonparametric estimation of Shannon's index of diversity when there are unseen species in the sample. Environmental and Ecological Statistics. 2003;10:429–443. [Google Scholar]
- 32.Giudicelli V, Chaume D, Lefranc MP. IMGT/GENE-DB: a comprehensive database for human and mouse immunoglobulin and T cell receptor genes. Nucleic Acids Res. 2005;33:D256–D261. doi: 10.1093/nar/gki010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37:D1006–D1012. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amrani A, Serra P, Yamanouchi J, Trudeau JD, Tan R, Elliott JF, Santamaria P. Expansion of the antigenic repertoire of a single T cell receptor upon T cell activation. J Immunol. 2001;167:655–666. doi: 10.4049/jimmunol.167.2.655. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman SM, Takaki T, Han B, Santamaria P, Serreze DV, DiLorenzo TP. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J Immunol. 2004;173:6727–6734. doi: 10.4049/jimmunol.173.11.6727. [DOI] [PubMed] [Google Scholar]
- 36.Kerry SE, Buslepp J, Cramer LA, Maile R, Hensley LL, Nielsen AI, Kavathas P, Vilen BJ, Collins EJ, Frelinger JA. Interplay between TCR affinity and necessity of coreceptor ligation: high-affinity peptide-MHC/TCR interaction overcomes lack of CD8 engagement. J Immunol. 2003;171:4493–4503. doi: 10.4049/jimmunol.171.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maile R, Wang B, Schooler W, Meyer A, Collins EJ, Frelinger JA. Antigen-specific modulation of an immune response by in vivo administration of soluble MHC class I tetramers. J Immunol. 2001;167:3708–3714. doi: 10.4049/jimmunol.167.7.3708. [DOI] [PubMed] [Google Scholar]
- 38.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 39.Yang W, Hussain S, Mi QS, Santamaria P, Delovitch TL. Perturbed homeostasis of peripheral T cells elicits decreased susceptibility to anti-CD3-induced apoptosis in prediabetic nonobese diabetic mice. J Immunol. 2004;173:4407–4416. doi: 10.4049/jimmunol.173.7.4407. [DOI] [PubMed] [Google Scholar]
- 40.Anderson B, Park BJ, Verdaguer J, Amrani A, Santamaria P. Prevalent CD8(+) T cell response against one peptide/MHC complex in autoimmune diabetes. Proc Natl Acad Sci U S A. 1999;96:9311–9316. doi: 10.1073/pnas.96.16.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong FS, Moustakas AK, Wen L, Papadopoulos GK, Janeway CA., Jr Analysis of structure and function relationships of an autoantigenic peptide of insulin bound to H-2K(d) that stimulates CD8 T cells in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 2002;99:5551–5556. doi: 10.1073/pnas.072037299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker C, Petrich de, Marquesini LG, Bishop AJ, Hedges AJ, Dayan CM, Wong FS. Human CD8 responses to a complete epitope set from preproinsulin: implications for approaches to epitope discovery. J Clin Immunol. 2008;28:350–360. doi: 10.1007/s10875-008-9177-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.