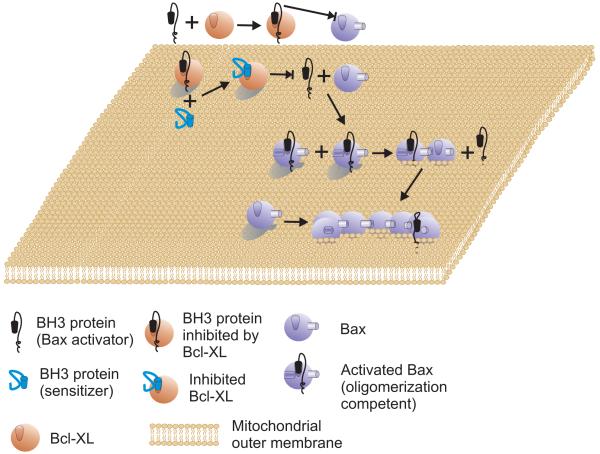
Figure 1.
The Direct Activation Model
An activator protein (black) such as tBid that contains a BH3 domain can bind to and activate Bax (powder blue). Activation of Bax leads to integration in the MOM and oligomerization. Oligomerization of Bax may displace the activator. Alternatively, one activated Bax may recruit additional Bax and therefore, there need be only one activator for each oligomer. Bak is predicted to function similarly but begins bound to the surface of MOM. Membrane permeabilization is inhibited by anti-apoptosis proteins (such as Bcl-XL shown) binding to the activator protein. Once bound to Bcl-XL the activator protein can no longer bind to or activate Bax. Sensitizer proteins compete with activator proteins for binding to Bcl-XL. Therefore, if the affinity for Bcl-XL or abundance of the sensitizer is greater than the activator it will displace it from Bcl-XL whereupon the activator can again bind to Bax.