Figure 3.
The Embedding Together Model - Initiation of membrane permeabilization.
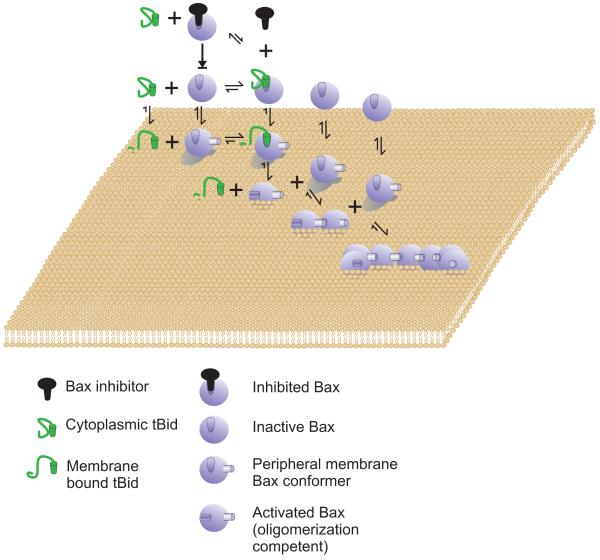
Bax is most frequently an inactive cytoplasmic protein. The binding affinity for cytoplasmic Bax for cytoplasmic BH3 proteins (tBid is shown) is low and cytoplasmic Bax is in equilibrium with form that binds with relatively low affinity to the surface of membranes. Therefore, other relatively low affinity interactions that might not survive immunoprecipitation can inhibit Bax in the cytoplasm by shifting the equilibrium away from membrane binding. Bax binds to membranes peripherally concomitant with a conformational change in Bax that increases the affinity of Bax/tBid interaction. In the diagram tBid is the only activator shown however, the model presupposes BH3 only proteins and other proteins that interact with Bax both in the cytoplasm and on the membrane can modulate one or more of the equilibriums shown. In the case of tBid the affinity for membranes is high and binding to membranes results in a conformation change that increases tBid affinity for Bax. The interaction between membrane bound tBid with peripherally membrane bound Bax triggers insertion of Bax into the membrane. The integral membrane form of Bax can recruit additional Bax proteins and oligomerize to permeabilize the outer mitochondrial membrane. The only step that is effectively irreversible is integration into the membrane. The model does not require that any one pro-apoptotic protein perform all of the functions ascribed to tBid, but rather envisions that most will perform only a subset of them. Bak is proposed to function similarly to Bax except that it is expected to bind the different modulators (e.g. tBid) with different affinities, its inactive conformation is constitutively membrane bound and is thus envisioned to be functionally similar to the peripheral membrane form of Bax.