In the present study, the impact of tumor-associated macrophages on retinal tumor growth in LHBETATAG mice was evaluated.
Abstract
Purpose.
To determine the distribution of tumor-associated macrophages (TAMs) during retinoblastoma tumor development, examine the contribution of bone marrow–derived TAMs in retinoblastoma tumors, and evaluate the supportive role of TAMs in tumor growth in a transgenic retinoblastoma mouse model.
Methods.
The time course of macrophage infiltration in transgenic retinoblastoma tumors was assessed by immunohistochemistry at different time points in tumorigenesis. The origin of TAMs in transgenic retinoblastoma tumors was determined by transplanting 107 bone marrow cells from green fluorescent protein (GFP)–positive 16-week-old mice into age-matched, irradiated LHBETATAG mice via tail vein injections. Macrophage depletion was performed by subconjunctival (SC) delivery of liposomal clodronate.
Results.
The density of TAMs increased from 4 to 12 weeks of age in mice with small to medium tumors (P = 0.037) and remained stable in the later stages of disease (i.e., 16 weeks old with large tumors; P = 0.20). In 16-week-old mice, 38% (2.5 ± 3.2 cells per 400× high-power field) of TAMs were GFP-positive, bone marrow–derived macrophages. Total TAM depletion was associated with a significant decrease in the expression levels of MMP-9 (P = 0.014) and mature vessels (P < 0.001) and a nonsignificant decrease in the density of neovessels (P = 0.94). The density of M2-polarized TAMs did not change significantly after TAM depletion (P = 0.68). After M1-polarized TAM depletion, the tumor burden increased (P = 0.056).
Conclusions.
This work extends understanding of the complex role that macrophages play in retinoblastoma. Macrophage modulation in the tumor microenvironment is a critical factor in retinoblastoma tumor progression.
Retinoblastoma is the most common primary intraocular malignancy in children,1,2 During the past decade, major improvements in screening and treatment have resulted in more than 95% long-term survival rates in the United States, which, in turn, has triggered research focusing on local tumor control and globe preservation. Current treatments are accompanied by significant local and systemic complications.3–5 Because of advanced disease, enucleation is performed in approximately 20% of cases of intraocular retinoblastoma.3,6
Tumors live in a milieu of cells and extracellular matrix that are essential for their growth.7 In some tumors, macrophages are an integral part of this microenvironment, having both tumor-promoting and -supporting roles in invasion, growth, angiogenesis, and metastasis. Tumor-associated macrophages (TAMs) are believed to support the developing tumor by expression of growth factors, chemokines, and extracellular matrix–remodeling enzymes.8 TAMs may be directly associated with tumor survival and growth by the expression of growth factors that are required for tumor cell proliferation, including EGF9 PDGF, TGF-α1, and bFGF.10 Conversely, TAMs may be involved indirectly in tumor growth by secreting the proteases essential for extracellular matrix remodeling, which in turn is necessary for tumor growth and angiogenesis. A protease that is often associated with macrophages is the gelatinase matrix metalloproteinase (MMP)-9, which potentiates tumor growth by promoting extracellular matrix remodeling and angiogenesis. For instance, supporting evidence has shown that macrophage-secreted MMP-9 is essential for the growth of ovarian tumors.11
Novel therapeutic modalities are needed for the treatment of retinoblastoma, since current therapies are associated with significant morbidity and potential mortality.6,12,13 Macrophage modulation in the tumor microenvironment is a feasible therapeutic strategy that requires understanding of the role macrophages play in retinoblastoma, particularly their specific contribution to the alteration of the tumor microenvironment that ultimately results in tumor development. The purposes of the study were to determine the distribution of TAMs in retinoblastoma tumor development, examine the contribution of bone marrow–derived TAMs in retinoblastoma tumors, and evaluate whether TAMs have a growth-supportive role in retinoblastoma tumor growth in a transgenic murine model.
Materials and Methods
LHBETATAG Mouse Model for Retinoblastoma
The study protocol was approved by the University of Miami Institutional Animal Care and Use Review Board Committee and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The LHBETATAG transgenic mouse model used in this study is characterized elsewhere.14 This animal model develops bilateral multifocal retinal tumors that are stable and grow at a predictable rate (i.e., tumor at 4 weeks is undetectable, at 8 weeks is small, at 12 weeks is medium, and at 16 weeks is large).15
LHBETATAG Retinal Tumor Growth, TAMs, and MMP-9 Activity
LHBETATAG retinal tumor samples were obtained from enucleations performed on LHBETATAG transgenic mice at 4, 8, 12, and 16 weeks of age (n = 14 per time point). The mice were euthanatized with CO2, and the eyes were enucleated. Macrophages and MMP-9 activity were detected by immunohistochemistry.
Bone Marrow Transplants
As we were interested in determining the contribution of bone marrow cells to the intraocular TAM population, bone marrow from mice expressing green fluorescent protein (GFP) were transplanted into irradiated LHBETATAG mice. LHBETATAG mice (n = 10; 10-week-old) harboring small to medium tumors were irradiated (950 cGy, single dose) to destroy their own bone marrow. (This dose of radiation does not affect retinal tumors.16,17) Donor bone marrow was obtained from T-cell depleted, age-matched GFP-C57bl/6/BALBc mice (n = 15; The Jackson Laboratory, Bar Harbor, ME). Donor mice were euthanatized by CO2 inhalation. Femurs were dissected immediately postmortem and bone marrow extracted by flushing with medium (RPMI 1640 culture medium, containing 2.5% 1 M HEPES and 1% gentamicin) inside the diaphyseal channel with a 27-gauge needle. Bone marrow cells were then homogenized, filtered, centrifuged, and resuspended. Bone marrow cells (107 cells) were transplanted into the irradiated, 10-week-old LHBETATAG mice via tail vein injections performed in mice under anesthesia (intramuscular administration of ketamine hydrochloride [25 mg/kg] and xylazine [5 mg/kg]). Irradiated mice (n = 5) and nonirradiated mice (n = 5) that did not receive transplanted bone marrow served as control groups. Blood components were allowed to reconstitute for 6 weeks, and then the mice were euthanatized by CO2 inhalation, and their eyes were enucleated for evaluation. GFP-positive cells that infiltrated tumors were identified by immunofluorescence.
Tumor size in chimeric mice comprised 40.3% of the globe space (as measured by the largest cross-sectional area in 60 serial hematoxylin–eosin [H&E]-stained sections); tumor size in untreated, nonirradiated litter-matched control subjects comprised 54.5% of the globe. Tumor size was not significantly altered by the dose of radiation.
Pharmacologic Depletion of Circulating Macrophages
The contribution of blood-derived macrophages to the tumor cell population was measured by counting the F4/80 or Iba-1 stained cells after pharmacologic depletion of the macrophages. Macrophage depletion was performed by subconjunctival (SC) delivery of clodronate encapsulated in liposomes (CL2MDP-lip; 0.25 μg SC). Animals (n = 7) were treated biweekly starting at 10 weeks of age for a total of 6 weeks, and were analyzed at 16 weeks of age. The control group (n = 7) received SC administration of PBS-liposomes. The effective depletion of macrophages was confirmed by flow cytometry of circulating monocytes and immunohistochemistry of TAMs.
Three delivery schedules (n = 7 per group) were initially used to optimize macrophage depletion and tumor burden reduction (2, 4, and 6 injections of either CL2MDP-lip or PBS-liposomes; 0.25 μg SC) for 6 weeks, starting at 10 weeks of age.
Tumor Burden Measurements
The eyes were sectioned serially and processed for standard H&E staining. Microscopic images of H&E-stained sections (50 sections; 8-μm sections per eye) were obtained with a digital camera at a magnification of 400×. The section of the eye containing the largest cross-sectional tumor area was chosen for analysis. Tumor boundaries were traced with the use of image-analysis software (Image Pro Express; Media Cybernetics, Silver Spring, MD). Tumor areas for all eyes were averaged, yielding an average area for each group. The tumor burden was expressed as the tumor/globe ratio by dividing the tumor area by the area of the globe, to normalize the data, as previously described.18
Immunofluorescence
Eyes were processed in sucrose (30%, 20%, and 10% for 30 minutes each), fixed with 4% paraformaldehyde (2 hours; 25°C), frozen in OCT (−80°C), and serially sectioned (8 μm). Tumor-infiltrating GFP cells were identified by immunofluorescence. Immunohistochemical analyses were performed on the samples, to detect macrophages with F4/80 (1:500; Serotec, Raleigh, NC) or Iba-1 (1:1000; Waco, Richmond, VA); total blood vessels with Alexa Fluor 568-conjugated lectin (Bandeira simplicifolia, a pan-endothelial binding agent; 1:1000; Invitrogen, Carlsbad, CA); mature vessels with α-SMA Cy3 conjugate (1:3000; Sigma Chemical Co., St. Louis, MO); neovessels with anti-endoglin (CD105 [clone SN6], 1:500; Abcam, Cambridge, MA); hypoxia with pimonidazole delivered via intraperitoneal (IP) 2 hours before enucleation (1:1000; Chemicon, Temecula, CA); Müller glia cells with c-FOS (1:250; Oncogene, San Diego, CA); MMP-9 with rabbit anti-MMP-9 polyclonal antibody (1:500; Abbiotec, LLC, San Diego, CA); and M2-polarized macrophages with CD-163 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA).19 Alexa Fluor 568 goat anti-mouse, 546 goat anti-rabbit, and 488 donkey anti-mouse were used as secondary antibodies (1:500; Invitrogen). The secondary antibodies were used alone as negative controls for nonspecific binding. Cell nuclei were stained for 5 minutes with 4′,6′ diamidino-2-phenylindole (DAPI, 1:5000; Invitrogen).
Serial cross sections of the tumors were examined for the presence of the different markers with an upright fluorescence microscope (BX51; Olympus American Inc., Melville, NY). All images were obtained at 200× magnification, with different filters used for the DAPI and Alexa Fluor 488 and 568 signals. Measured parameters (e.g., the number of GFP-labeled cells and immunostaining intensities) were evaluated as the average from at least five different adjacent sections per tumor per eye. The results from all the different sections were averaged. To analyze the co-staining of two markers, we determined the proportion of cells that were immunoreactive for F4/80 or Iba-1 and were also labeled with the other markers (e.g., total blood vessels, Müller glia cells, MMP-9). Areas of interest within the LHBETATAG retinal tumors were selected blindly by using DAPI staining. Only cells that had a nucleus clearly labeled with DAPI were incorporated in the analyses.
Statistical Methods
The average densities of macrophages, blood vessels, and expression levels of MMP-9 within each tumor were compared using two-sided, paired t-tests. Results were considered significant if P ≤ 0.05.
Results
LHBETATAG Retinal Tumor Growth and TAMs
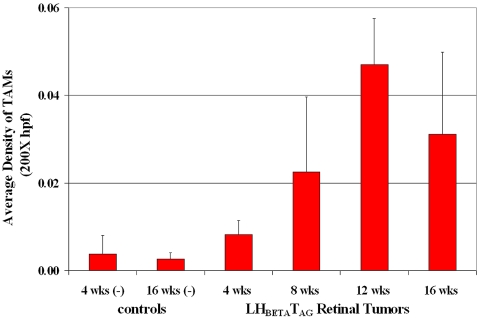
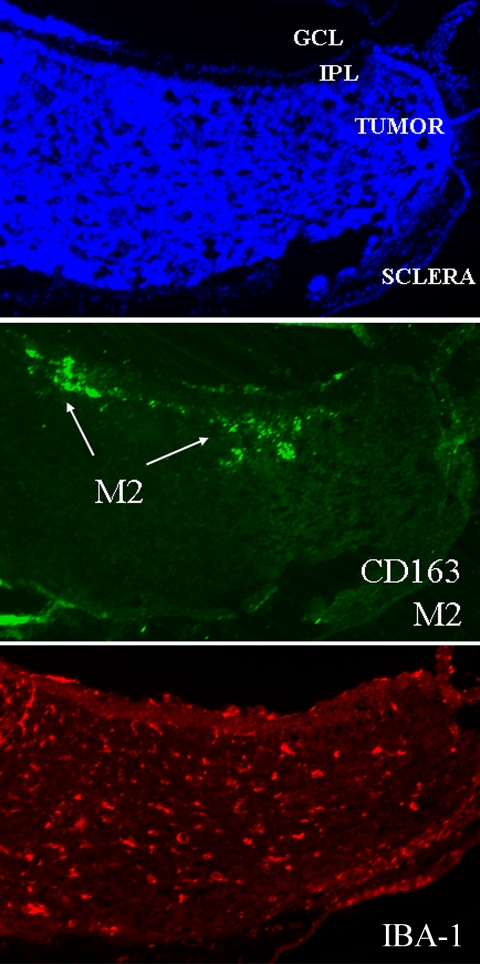
As we wanted to determine the distribution of macrophages during retinoblastoma development, the density of TAMs was compared with tumor growth. We observed that the density of TAMs in LHBETATAG retinal tumors was directly proportional to tumor size. TAMs were detected in the tumor microenvironment of the LHBETATAG mice as early as the 4-week stage (Fig. 1). At this time point, the density of TAMs was significantly higher in the retinal regions composed of microscopic tumor foci (rosettelike) than in the normal retina (P = 0.013). The density of TAMs showed a steady statistically significant increase from 4 to 12 weeks of age (i.e., small to medium tumors, respectively; P = 0.037), and a steadiness between 12 and 16 weeks of age (i.e., medium to large tumors, respectively; P = 0.20); 1.3%, 5.4%, and 3.7% of the tumor area was composed of macrophages in the small, medium, and large tumors, respectively. In the LHBETATAG retinal tumors, 62.0% of TAMs showed co-localization with blood vessels; 48.6% of TAMs co-localized with neovessels and 13.4% with mature blood vessels. TAMs not associated with vessels showed a co-localization with activated Müller glia (58%). A very small portion of TAMs was found to be associated with hypoxic tumor areas (<5%). In addition, more than 51% of TAMs co-localized with the expression of MMP-9. CD163-positive, M2-polarized macrophages represented 55% of the total TAMs at 16 weeks of age, localized mainly in the tumor apex.
Figure 1.
TAMs were recruited or activated early in the disease course. They were detected in the tumor microenvironment of the LHBETATAG mice as early as 4 weeks of age. At this time point, the density of TAMs was significantly higher in the retinal regions composed of microscopic tumor foci (rosettelike) than in the normal retina (P = 0.013). The density of TAMs showed a steady statistically significant increase in size from 4 to 12 weeks of age, with small to medium tumors, respectively (P = 0.037), and a continued steady increase in size in the later stages of the disease (i.e., from 12 to 16 weeks of age, with large tumors present at 16 weeks of age; P = 0.20): 1.3%, 5.4%, and 3.7% of the tumor area was composed of macrophages in the small, medium, and large tumors, respectively.
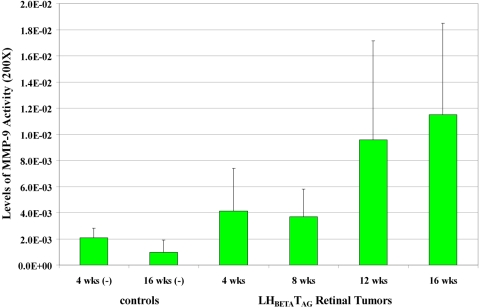
As we wanted to determine the expression of MMP-9 activity levels in the tumor at different time points of LHBETATAG retinal tumor development, the levels of MMP-9 activity were compared to tumor growth. We observed that the levels of MMP-9 activity in LHBETATAG retinal tumors were directly proportional to tumor size (Fig. 2). MMP-9 activity levels significantly increased from 4 to 16 weeks of age (P = 0.042). These levels did not significantly differ in the LHBETATAG retinal tumors from the wild-type, negative control tumors at 4 weeks of age (i.e., small tumors; P = 0.33); however, MMP-9 levels significantly increased in the LHBETATAG retinal tumors when compared with the wild-type, negative control tumors at 8 weeks of age (i.e., small to medium tumors; P = 0.035), 12 weeks of age (medium tumors; P = 0.016), and 16 weeks of age (i.e., large tumors; P = 0.019).
Figure 2.
Levels of MMP-9 activity at different time points of tumor development in LHBETATAG retinal tumors. MMP-9 activity levels significantly increased from 4 to 16 weeks of age (P = 0.042). MMP-9 activity levels did not significantly differ in the LHBETATAG retinal tumors from that in the wild-type, negative control tumors at 4 weeks of age (i.e., small-size tumors; P = 0.33); however, these levels significantly increased in the LHBETATAG retinal tumors when compared with the wild-type, negative control tumors at 8 (small to medium tumors; P = 0.035), 12 age (medium tumors; P = 0.016), and 16 (large tumors; P = 0.019) weeks of age.
Bone Marrow Transplantation
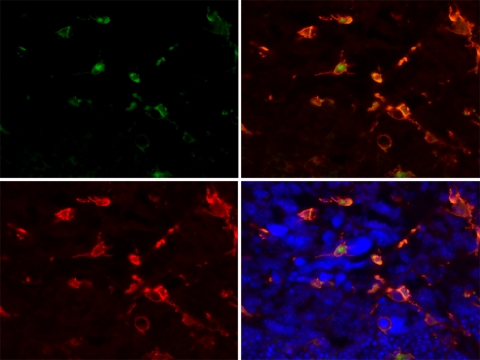
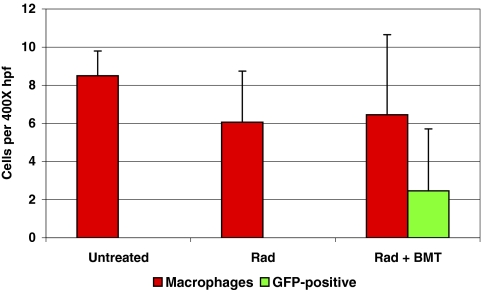
To determine the contribution of bone marrow–derived TAMs in retinoblastoma tumors, tumor burden, and TAMs were compared between nonirradiated, irradiated, and irradiated+bone marrow transplant–recipient mice (GFP-chimeric mice). Irradiated mice survived 3 weeks after irradiation in the absence of bone marrow transplants. Chimeric mice survived until killed at the experimental endpoint, 6 weeks after treatment. Bone marrow–derived macrophages were present in small retinal tumors harbored by 12-week-old LHBETATAG mice. In advanced tumors (i.e., 16 weeks of age), 38% (2.5 ± 3.2 cells per 400× hpf) of the macrophage population was GFP positive, representing bone marrow–derived macrophages (Figs. 3, 4). The number of macrophages in tumors from irradiated mice and GFP-chimeric mice (6.06 ± 2.68 and 6.45 ± 4.20, respectively) was slightly lower than the number in untreated control animals (8.50 ±1.29). However, the difference was not significant (P = 0.15).
Figure 3.
Bone marrow–derived macrophages are present in advanced LHBETATAG retinal tumors. The image shows a retinal tumor from a 16-week-old LHBETATAG chimera with numerous GFP-positive cells (green, top left). The tumors were stained with F4/80, to visualize microglia/macrophages (red, bottom left). Right: merged images; bottom: DAPI-stained nuclei. Magnification, ×400.
Figure 4.
A fraction of TAMs in LHBETATAG retinal tumors were bone marrow–derived. Retinal tumors in 16-week-old LHBETATAG mice had 8.50 ± 1.29 macrophages per 400× high-power field (hpf), irradiated mice had 6.06 ± 2.68, and chimeras had 6.45 ± 4.20. The difference in macrophage density between the untreated, nonirradiated control group and the irradiated control group is not significant (P = 0.15). GFP-positive cells in chimeras comprised 38% of the macrophage population (2.5 ± 3.2 cells per 400× hpf). Bars, SD (n = 5; n = 10 for chimeras).
Pharmacologic Depletion of Circulating Macrophages
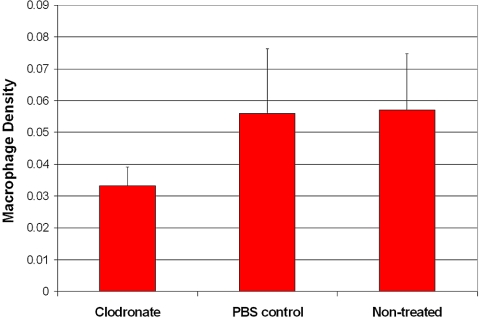
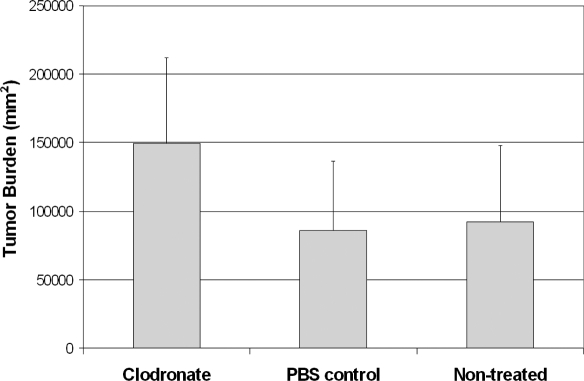
To establish whether the presence of TAMs contributes to tumor growth, we studied mice with and without macrophage depletion. There was a significant decrease in the number of TAMs in the group treated with liposomal clodronate compared with the number in the group treated with PBS liposomes only (P = 0.037; Fig. 5); there was no significant difference between the PBS and the nontreated control groups (P = 0.64). After TAM depletion, the tumor burden increase was of borderline statistical significance (P = 0.056; Fig. 6); there was no significant difference between the PBS control and the nontreated control groups (P = 0.52).
Figure 5.
Macrophage depletion with clodronate liposomes. There was a significant decrease in TAMs in the group treated with clodronate-encapsulated liposomes compared with the group treated with PBS-encapsulated liposomes (P = 0.037). The difference between the PBS-treated and the nontreated control groups was not significant (P = 0.64).
Figure 6.
The tumor burden increased after macrophage depletion. There was a borderline statistically significant increase in tumor burden in the group treated with clodronate-encapsulated liposomes compared with the group treated with PBS-encapsulated liposomes (P = 0.056). There was no significant difference between the PBS and the nontreated control groups (P = 0.52).
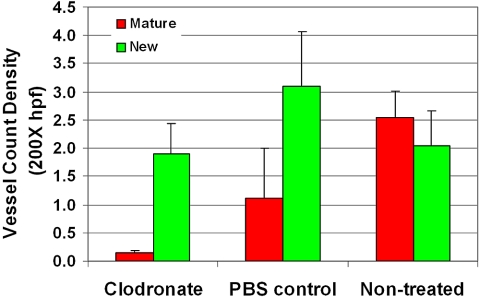
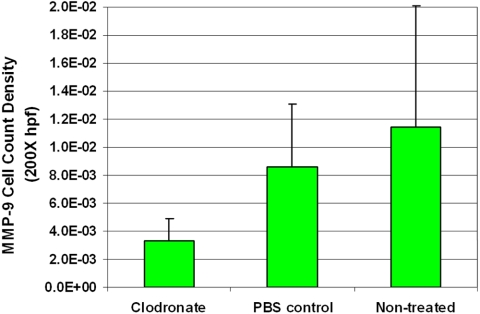
Moreover, a trend of neo- and mature vessel decrease was noted after TAM depletion. The difference between TAM-depleted tumors and nontreated control tumors was statistically significant for mature vessels (P < 0.001), but not for neovessels (P = 0.94; Fig. 7). Furthermore, the expression of MMP-9 decreased significantly (P = 0.014; Fig. 8). When investigating the TAM subtypes present in the tumor, we observed that CD163-positive macrophages (which identifies the M2-polarized, tumor-promoting macrophage type) were mainly located in the tumor apex, and that their density did not change after TAM depletion (P = 0.68; Fig. 9).
Figure 7.
Neovessel and mature vessel density did not change after macrophage depletion. A trend toward a decrease in neovessels and mature vessels was noted after depletion of TAMs. The difference between TAM-depleted tumors and the nontreated control is statistically significant for mature vessels (P < 0.001), but not for neovessels (P = 0.94).
Figure 8.
The expression levels of MMP-9 decreased after macrophage depletion. There was a significant decrease in the density of MMP-9 in the group treated with clodronate-encapsulated liposomes compared with the group treated with PBS-encapsulated liposomes (P = 0.014).
Figure 9.
M2-polarized macrophages were present in the apex. M2-polarized macrophages were present only in the apical regions of the tumor. The density of this type of macrophage did not change after macrophage depletion (P = 0.68).
Discussion
The retina contains two types of macrophages: resident microglia and bone marrow–derived macrophages. Both types have been implicated in traumatic, infectious, and degenerative disorders of the central nervous system, including tumors.20 The distinction between resident microglia and bone marrow– derived macrophages may have profound implications for therapy—for instance, when drugs can be used to modulate macrophage recruitment or function. In a rat model of prostate cancer, treatment with immunomodulators resulted in tumor reduction by inhibition of macrophage infiltration.21 It is noteworthy that in choroidal neovascularization, bone marrow–derived macrophages, and not microglia, primarily infiltrate the retina and activate Müller cells, leading to disease progression.22
TAMs constitute a significant proportion of the tumor mass in most tumors.23 Their presence correlates with poor prognosis in 80% of tumors evaluated, including breast, prostate, ovarian, and cervical cancers24 and the intraocular malignancy uveal melanoma.25 However, their presence has been indicative of a good prognosis in other tumors, such as colorectal cancers26 and intraocular embryonic cell tumors grown in mice.27 Since macrophage subtypes are known to communicate with their environment, a better understanding of their presence and distribution in the tumor microenvironment may explain this variation. Macrophages can be “educated” by adjacent tumor cells to adopt a trophic role in tumor development.23 This effect is accomplished by a change in the functional program, or polarization, of the recruited macrophage.28,29 M1-polarized macrophages produce tissue-destructive reactions centered on the vascular endothelium by stimulating cytotoxic activity on tumor cells. In contrast, M2-polarized macrophages, considered the anti-inflammatory phenotype, produce growth and angiogenic factors, in addition to proteases such as MMP-9 and -2 that degrade the extracellular matrix.30 M2-polarized macrophages have been associated with tumor growth in many tumors.
Retinoblastoma tumors display signs of damage to the blood–retinal barrier, such as leukocytic infiltrates. In these tumors, macrophages are often localized adjacent to tumor vessels, usually embedded in the perivascular space.31 The presence and position of these macrophages in retinoblastoma tumors may be indicative of a vital role in this disease. If so, macrophage-targeting agents, or anti-inflammatory agents, may be of significant therapeutic benefit in treating retinoblastoma tumors. Macrophages are interesting targets for retinoblastoma therapy, given their potential pleiotropic role in tumor development and their stable genome.
In this study, the presence and density of macrophages in the tumor microenvironment of LHBETATAG retinal tumors were analyzed during tumor development. TAMs were detected in retinal tumors in the eyes of LHBETATAG mice at all time points of tumor development. The TAMs were present during early disease in abnormal retinal regions characterized by rosette formation. The density of these macrophages steadily increased with tumor progression, reaching a steady state in the later stages of disease (Fig. 1). Knowledge of the exact timing of macrophage recruitment allowed the optimization of bone marrow transplant schedules for the subsequent experiment.
In this study, we evaluated the relationship of macrophages to tumor vasculature. A fraction of TAMs (62.0%) are tightly associated with the vasculature of LHBETATAG tumors. TAMs that were not associated with vessels co-localized with activated Müller glia (58%). In addition, we have been studying the effects of hypoxic regions on tumor burden, which present with slow-growing chemoresistant cells that are most prevalent in advanced disease.32 Of interest, macrophages do not co-localize to hypoxic tumor areas, suggesting that hypoxia is not a major mechanism of macrophage recruitment to retinal tumors in this model.
TAMs may be involved in the development of many tumors by releasing proteases such as MMP-9 that are critical for ECM remodeling. MMP-9 potentiates tumor growth by promoting ECM remodeling, tumor growth, and angiogenesis.11 In the present study, our data revealed that MMP-9 co-localizes with macrophages (51%), suggesting that macrophages are responsible for a large fraction of MMP-9 expression in these tumors. In our work, the expression levels of MMP-9 were low during early stages of tumor development and increased with tumor growth, becoming most prevalent during the later stages of tumor growth in the LHBETATAG retinal tumors (Fig. 2). This pattern is very similar to the pattern identified in this study regarding macrophage density and tumor growth.
Since the distinction between resident microglia and bone marrow–derived macrophages may have profound implications for therapy,21 we transplanted bone marrow–derived, GFP-positive cells into our LHBETATAG transgenic mouse model. The results showed that the bone marrow–derived macrophages infiltrated LHBETATAG retinal tumors early in the disease course. The number of bone marrow–derived macrophages increased to 38% of total TAMs in advanced disease. Furthermore, we hypothesize that the number of bone marrow–derived macrophages is underestimated due to the possible presence of such cells either before conducting the bone marrow transplantations in 10-week-old mice or before the enumeration of the cells at 16 weeks of age.
The role that macrophages play in intraocular tumor growth was evaluated by depleting intraocular macrophages by SC delivery of clodronate liposomes, which selectively deplete macrophages by inducing apoptosis in the macrophages that engulf them. The total number of macrophages in the LHBETATAG retinal tumors was significantly reduced, even though not all the macrophages were depleted. It is noteworthy to mention that clodronate liposomes were delivered via subconjunctival injections, not intraperitoneal injections, which means that intraocular macrophages were preferentially depleted. Since the tumor microenvironment is dynamic and some macrophages may infiltrate continually or be transformed by the angiogenic switch,33 we expected to find that not all the intraocular macrophages were depleted after subconjunctival injections of clodronate liposomes. In addition, after macrophage depletion, the macrophages found in the tumor may be anatomically or histopathologically present, but they may not be functional.34–36 After a significant depletion of the intraocular macrophages in LHBETATAG retinal tumors (Fig. 5), the tumor burden did not decrease but increased with borderline statistical significance (Fig. 6). Of importance, these data suggest that in retinoblastoma, unlike 80% of solid tumors, TAMs help control tumor burden rather than promote it.
Our data also show that the expression levels of MMP-9 decreased significantly after macrophage depletion (Fig. 8). Tumor blood vessels have been reported to express MMP-9 protein.11 However, in this study, local macrophage depletion did not influence the density of neovessels significantly, while causing a significant decrease in mature blood vessels (Fig. 7). These results support our hypothesis that TAMs are responsible for a large fraction of the expression levels of MMP-9 in LHBETATAG retinal tumors.
The finding in this study that macrophage depletion resulted in a trend of tumor burden increase while causing a decrease in MMP-9 activity were in contradistinction with our expectations, since MMP-9 activity has been shown to be directly associated with angiogenesis and tumor growth.37 Nevertheless, these results may not be surprising, since MMPs (e.g., MMP-9, -2) are constantly upregulated in the tumor microenvironment. Other MMPs such as MMP-2 have been found to be associated with angiogenesis and tumor growth as well as MMP-9.37 It is possible that the increased tumor burden in the absence of MMP-9 was caused by the upregulation of other MMPs, such as MMP-2, or by other unrelated microenvironmental factors.
To further explore the relationship of TAMs and transgenic retinoblastoma growth, we evaluated the phenotype of the TAMs. In the present study, 55% of TAMs were M2-polarized and were only present in the apical regions of the tumor (Fig. 9). The absolute number of M2-polarized macrophages did not change after macrophage depletion with clodronate liposomes. That the density of M2-polarized tumor-promoting macrophages remains stable and there is a significant decrease in total TAM density suggests that the M1-polarized macrophage population was preferentially depleted by SC-administered clodronate liposomes. Consistent with previous findings in other tumors,30 the reduction of M1-polarized macrophages, which usually play a role in tissue destruction and tumor rejection, seemed to result in tumor growth. The continued presence of M2-polarized macrophages may also have contributed to the continued presence of blood vessels that play a role in tumor growth.
The use of the LHBETATAG transgenic mouse model in the present study presents several caveats for the translation of immunotherapy into treatment of human retinoblastoma. Findings from this study of the LHBETATAG transgenic mouse model may not be directly applicable to treating humans, because of the genetic, biochemical, and immunologic differences that exist between mice and humans. Moreover, immunomodulation as a treatment modality may be difficult to assess in humans. Finally, it is unclear whether the phenotypic differentiation of macrophages in humans occurs in a fashion similar to that in animal models. However, the use of this animal model is essential for the understanding of the ontogeny, pathogenesis, and treatment of retinoblastoma in humans.
In this study, modulation of the total TAMs population did not reduce tumor burden. Nonetheless, targeted modulation to potentially increase M1-polarized and decrease M2-polarized macrophages may remain a viable therapeutic strategy that should be explored in future studies. This potential therapy is of particular importance in retinoblastoma because current therapies are associated with significant morbidity and potential mortality.6,12,13 A better understanding of TAMs in retinoblastoma and the potential for immunotherapy may lead to novel therapeutic strategies in the LHBETATAG transgenic mouse model, which can be further translated to treating children with retinoblastoma.
Footnotes
Supported by National Institutes of Health Center Grants R01 EY013629, R01 EY12651, and P30 EY014801; the American Cancer Society, the Sylvester Comprehensive Cancer Center; and an unrestricted grant to the University of Miami from Research to Prevent Blindness, Inc.
Disclosure: Y. Piña, None; H. Boutrid, None; T.G. Murray, None; M.J. Jager, None; C.M. Cebulla, None; A. Schefler, None; L.V. Ly, None; A. Alegret, None; M. Celdran, None; W. Feuer, None; M.-E. Jockovich, none
References
- 1.Pendergrass TW, Davis S. Incidence of retinoblastoma in the United States. Arch Ophthalmol 1980;98:1204–1210 [DOI] [PubMed] [Google Scholar]
- 2.Tamboli A, Podgor MJ, Horm JW. The incidence of retinoblastoma in the United States: 1974 through 1985. Arch Ophthalmol 1990;108:128–132 [DOI] [PubMed] [Google Scholar]
- 3.Abramson DH, Beaverson KL, Chang ST, Dunkel IJ, McCormick B. Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol 2004;122:1316–1323 [DOI] [PubMed] [Google Scholar]
- 4.Khelfaoui F, Validire P, Auperin A, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer 1996;77:1206–1213 [PubMed] [Google Scholar]
- 5.Shields CL, Shields JA, Baez KA, Cater J, De Potter PV. Choroidal invasion of retinoblastoma: metastatic potential and clinical risk factors. Br J Ophthalmol 1993;77:544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouic L, Aerts I, Lévy-Gabriel C, et al. Conservative treatments of intraocular retinoblastoma. Ophthalmology 2009;115(8):1405–1410.e2 [DOI] [PubMed] [Google Scholar]
- 7.Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell 2005;7:513–520 [DOI] [PubMed] [Google Scholar]
- 8.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263–266 [DOI] [PubMed] [Google Scholar]
- 9.O'Sullivan C, Lewis CE, Harris AL, McGee JO. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet 1993;342:148–149 [DOI] [PubMed] [Google Scholar]
- 10.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol 2005;167:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S, Van Arsdall M, Tedjarati S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst 2002;94:1134–1142 [DOI] [PubMed] [Google Scholar]
- 12.Berman EL, Donaldson CE, Giblin M, Martin FJ. Outcomes in retinoblastoma. 1974–2005: the Children's Hospital, Westmead. Clin Exp Ophthalmol 2007;35:5–12 [DOI] [PubMed] [Google Scholar]
- 13.Schefler AC, Cicciarelli N, Feuer W, Toledano S, Murray TG. Macular retinoblastoma: evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablation. Ophthalmology 2007;114:162–169 [DOI] [PubMed] [Google Scholar]
- 14.Windle JJ, Albert DM, O'Brien JM, et al. Retinoblastoma in transgenic mice. Nature 1990;343:665–669 [DOI] [PubMed] [Google Scholar]
- 15.Jockovich ME, Pina Y, Alegret A, Cebulla C, Feuer W, Murray TG. Heterogeneous tumor vasculature in retinoblastoma: implications for vessel targeting therapy. Retina 2008;28:S81–S86 [DOI] [PubMed] [Google Scholar]
- 16.Murray TG, Roth DB, O'Brien JM, et al. Local carboplatin and radiation therapy in the treatment of murine transgenic retinoblastoma. Arch Ophthalmol 1996;114:1385–1389 [DOI] [PubMed] [Google Scholar]
- 17.Murray TG, O'Brien JM, Steeves RA, et al. Radiation therapy and ferromagnetic hyperthermia in the treatment of murine transgenic retinoblastoma. Arch Ophthalmol 1996;114:1376–1381 [DOI] [PubMed] [Google Scholar]
- 18.Jockovich ME, Murray TG, Escalona-Benz E, Hernandez E, Feuer W. Anecortave acetate as single and adjuvant therapy in the treatment of retinal tumors of LH(BETA)T(AG) mice. Invest Ophthalmol Vis Sci 2006;47:1264–1268 [DOI] [PubMed] [Google Scholar]
- 19.Law SK, Micklem KJ, Shaw JM, et al. A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur J Immunol 1993;23:2320–2325 [DOI] [PubMed] [Google Scholar]
- 20.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res 2005;81:447–455 [DOI] [PubMed] [Google Scholar]
- 21.Joseph IB, Isaacs JT. Macrophage role in the anti-prostate cancer response to one class of antiangiogenic agents. J Natl Cancer Inst 1998;90:1648–1653 [DOI] [PubMed] [Google Scholar]
- 22.Caicedo A, Espinosa-Heidmann DG, Pina Y, Hernandez EP, Cousins SW. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res 2005;81:38–47 [DOI] [PubMed] [Google Scholar]
- 23.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71–78 [DOI] [PubMed] [Google Scholar]
- 24.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002;196:254–265 [DOI] [PubMed] [Google Scholar]
- 25.Makitie T, Summanen P, Tarkkanen A, Kivela T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci 2001;42:1414–1421 [PubMed] [Google Scholar]
- 26.Nakayama Y, Nagashima N, Minagawa N, et al. Relationships between tumor-associated macrophages and clinicopathological factors in patients with colorectal cancer. Anticancer Res 2002;22:4291–4296 [PubMed] [Google Scholar]
- 27.Boonman ZF, Schurmans LR, van Rooijen N, Melief CJ, Toes RE, Jager MJ. Macrophages are vital in spontaneous intraocular tumor eradication. Invest Ophthalmol Vis Sci 2006;47:2959–2965 [DOI] [PubMed] [Google Scholar]
- 28.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 2006;42:717–727 [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer 2004;40:1660–1667 [DOI] [PubMed] [Google Scholar]
- 30.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 2008;222:155–161 [DOI] [PubMed] [Google Scholar]
- 31.Madigan MC, Penfold PL. Human retinoblastoma: a morphological study of apoptotic, leukocytic, and vascular elements. Ultrastruct Pathol 1997;21:95–107 [DOI] [PubMed] [Google Scholar]
- 32.Boutrid H, Jockovich ME, Murray TG, et al. Targeting hypoxia, a novel treatment for advanced retinoblastoma. Invest Ophthalmol Vis Sci 2008;49:2799–2805 [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353–364 [DOI] [PubMed] [Google Scholar]
- 34.Slegers TP, Torres PF, Broersma L, van Rooijen N, van Rij G, van der Gaag R. Effect of macrophage depletion on immune effector mechanisms during corneal allograft rejection in rats. Invest Ophthalmol Vis Sci 2000;41:2239–2247 [PubMed] [Google Scholar]
- 35.Slegers TP, van der Gaag R, van Rooijen N, van Rij G, Streilein JW. Effect of local macrophage depletion on cellular immunity and tolerance evoked by corneal allografts. Curr Eye Res 2003;26:73–79 [DOI] [PubMed] [Google Scholar]
- 36.Slegers TP, van der Veen G, Hermans LJ, et al. Adhesion molecule expression in local-macrophage-depleted rats bearing orthotopic corneal allografts. Graefes Arch Clin Exp Ophthalmol 2003;241:432–438 [DOI] [PubMed] [Google Scholar]
- 37.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006;25:9–34 [DOI] [PubMed] [Google Scholar]