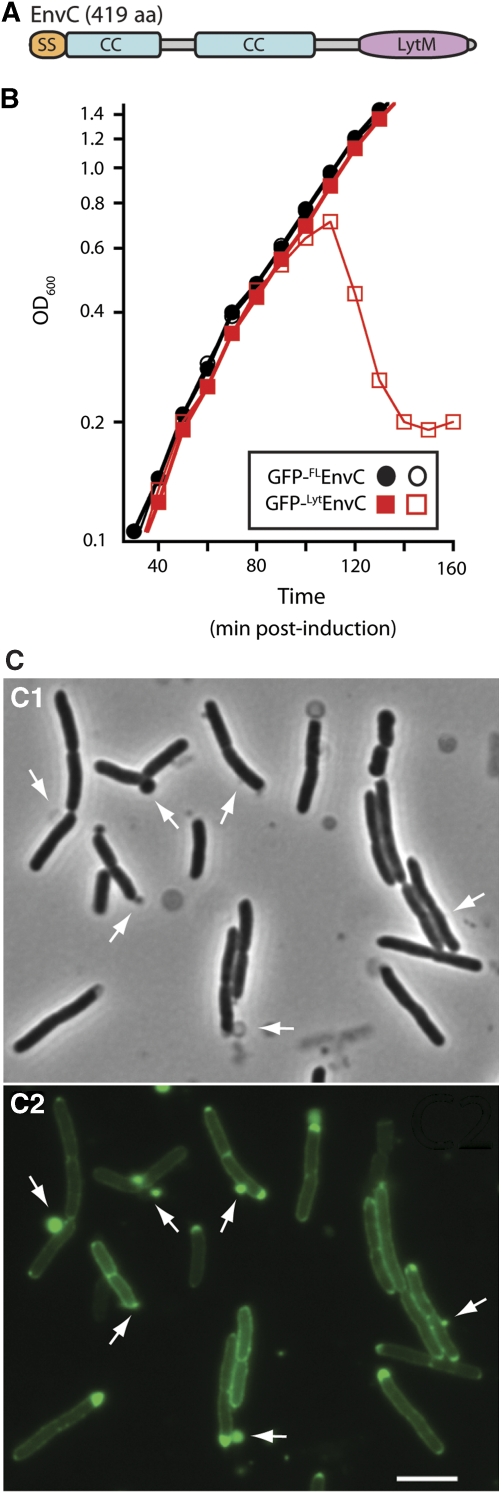
Figure 2.
The LytM domain of EnvC induces lysis. (A) Predicted domain structure of EnvC. SS, signal sequence; CC, coiled coil; LytM, LytM/lysostaphin/peptidase_M23 domain. (B) Wild-type (MG1655) cells harbouring the expression constructs pTU113 [Plac∷ssdsbA-gfp-flenvC] or pTU115 [Plac∷ssdsbA-gfp-lytenvC] integrated at the phage HK022 att site were grown in LB at 37°C to an OD600 of about 0.5. At t=0, the cultures were diluted into fresh LB with (open symbols) or without (closed symbols) 20 μM IPTG and growth was continued at 37°C. Induction of GFP-FLEnvC with much higher IPTG levels (250 μM–1 mM) also failed to affect cell growth (data not shown). Fusions encoded the signal sequence from dsbA for export and the GFP variant used was superfolder GFP (Pédelacq et al, 2006), which we have found is functional in the periplasm following Sec export (T Dinh, unpublished results). (C) Phase contrast (C1) and GFP-fluorescence (C2) micrographs of cells lysing as a result of GFP-LytEnvC production. Arrows highlight cell envelope defects, many of which correspond to regions with an enlarged periplasm as indicated by the accumulation of the exported GFP-LytEnvC fusion. Bar=4 μm. See Supplementary Figure S1C for images of cells before the onset of lysis.