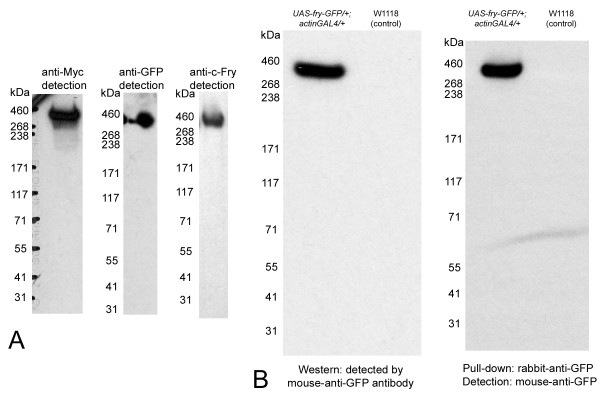
Figure 2.
Detection of intact Fry. (A) A single and very large protein is detected in Western blots of UAS-Myc-fry-GFP/+; actinGAL4/+ tissues probed with anti-Myc, anti-GFP or anti-c-fry antibody. The protein band migrates between marker proteins that are 268 kDa and 460 kDa. Consistent with its predicted molecular weight (418 kDa) the tagged Fry protein migrates to closer to the 460 kDa marker. (B) Fry can be detected after immunoprecipitation. The left panel shows a Western blot of extracts of 30 wing discs from UAS-Myc-fry-GFP/+; actin-GAL4/+ larvae or w1118 larvae (control lane) detected by anti-GFP antibody. A strong Fry signal is seen in the experimental lane but not in the control lane. The right panel shows similar extracts immunoprecipitated with rabbit anti-GFP antibody followed by detection using mouse anti-GFP antibody. The light line at the lower part of the film is caused by bending of the film and is not a western signal. Note the strong positive signal in the UAS-Myc-fry-GFP/+; actin-GAL4/+ lane showing that we were able to immunoprecipitate the large Fry protein.