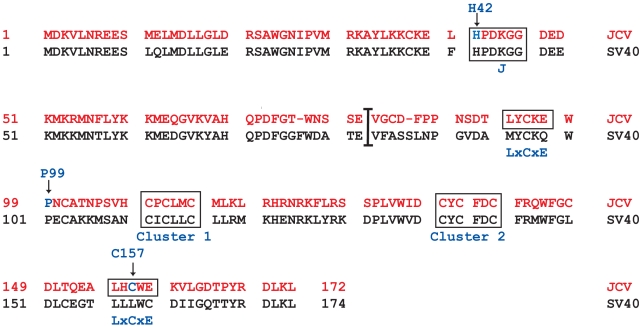
Figure 2. Comparison of JCV and SV40 tAg amino acid sequences.
The JCV tAg sequence containing 172 amino acids is indicated in red letters, and the 174 amino acid SV40 tAg is shown in black letters. The sequences following the vertical line comprise the unique C-terminal region of each protein. Two conserved cysteine (CxCxxC) clusters (Cluster 1, 2) that contribute to SV40 tAg binding to zinc ions, and possibly to PP2A, are noted in both sequences, as are two recently recognized LxCxE motifs in JCV tAg. Three amino acid residues are highlighted with a blue letter and arrow. Histidine 42 (H42) is a conserved residue in the HPDKGG hexapeptide motif of the J domain that spans most of the N-terminal region of tAg as well as the other JCV and SV40 early proteins; H42 is required for functional interaction with Hsc70. Proline 99 is within a conserved CxxxPxC sequence that influences SV40 tAg binding to PP2A. Cysteine 157 is the central residue of the newly recognized second LxCxE motif in JCV tAg.