Figure 3.
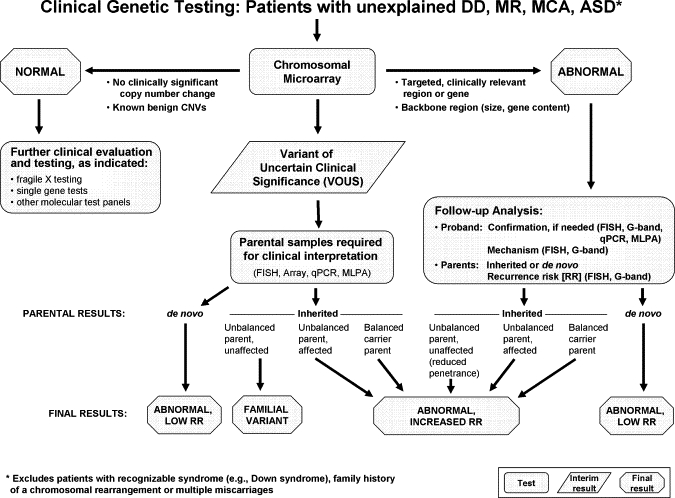
Algorithm for CMA Testing in Patients with Unexplained DD, MR, MCA, and ASD
This algorithm assumes that the patient does not present with features of a recognizable syndrome or metabolic disorder or that tests have been negative for a suspected disorder. The first-tier test is a chromosomal copy-number array or CMA. If no copy-number changes are identified, or if only known CNVs that are known to be benign are identified, this testing is considered “normal” (left side of figure), and further clinical evaluation is warranted to determine whether other testing should be pursued on the basis of the clinical presentation. If a CNV is detected within a known, clinically relevant region or gene, or if the CNV is in the genomic backbone and meets recommended size and gene content guidelines, then the result is considered a pathogenic CNV and “abnormal” (right side of figure). For these cases, follow-up analyses include confirmation studies and determination of the mechanism of imbalance in the proband and parental analysis to determine recurrence risk. All other results are considered VOUS until parental analysis is performed to aid in the final clinical interpretation. After the parental analyses of “abnormal” and “VOUS” results, final results may be classified into three major categories: familial variant, abnormal with a low recurrence risk (RR), or abnormal with an increased RR. In addition, the final interpretation may remain VOUS in some instances, even after parental testing.
