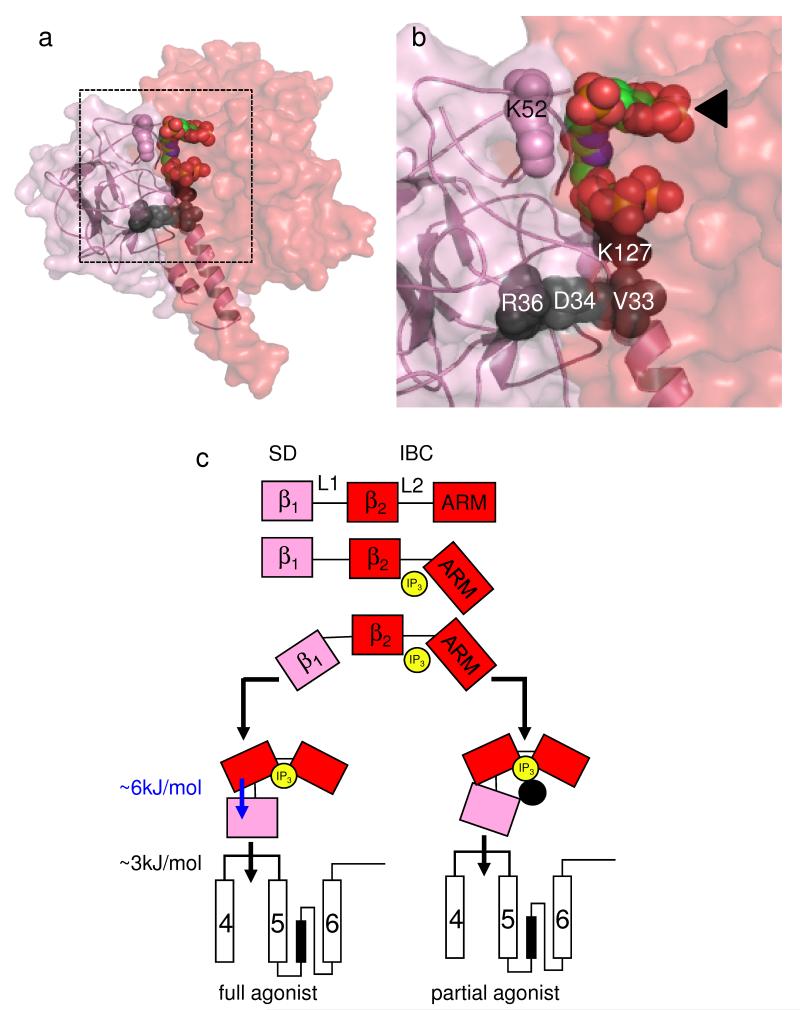
Figure 5.
IP3 binding to the IBC activates IP3R entirely via the SD. (a,b) Predicted relationship between the SD (pink) and IBC (red) with 2 bound. Residues within the SD that affect efficacy (V33, D34, R36 and K127) are shown in black (see Fig. 4a and Supplementary Fig. 3 online for details). The ineffective residue K52 is shown in pink. Panel b is an enlargement of the boxed area in panel a, with the IBC-bound IP3 moiety indicated by an arrow. (c) IP3 (yellow) rearranges the 2 domains of the IBC (β2 and ARM, red) around its L2 loop causing rearrangement of the SD (= β1, pink) around the L1 loop. The SD is then entirely responsible for transmitting conformational changes towards the pore, probably by directly interacting with the TMD4-5 loop of an adjacent subunit31,32. ΔG associated with rearranging the SD and its subsequent communication with the pore region is shown. Partial agonists effectively rearrange the IBC, but the inositol 2-O-substituent (or point mutations in the SD; black circle) disrupt the IBC-SD interface and so block communication with the SD. The latter is now less likely to contact the TMD4-5 loop, but once it makes contact the channel gates normally.