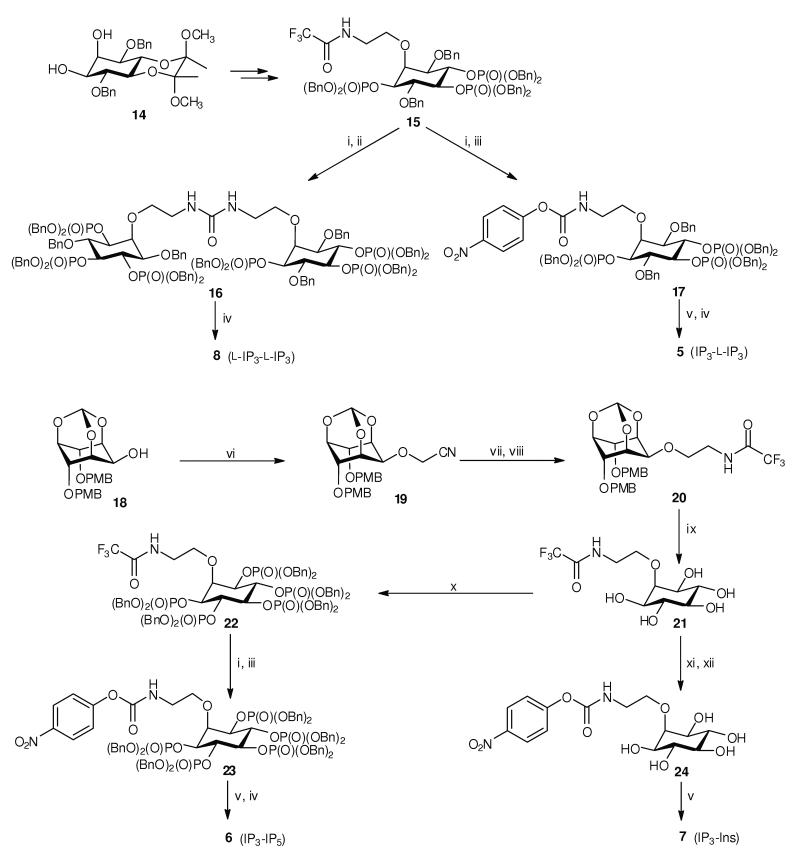
Scheme 1.
Syntheses of hetero-dimers 5, 6 and 7, and L-IP3 dimer 8. Dimers 5 and 8 were synthesized from L-IP3-based building block 15, obtained from diol 1448 (see Supplementary Methods online). The N-trifluoroacetyl protecting group was removed, generating an unstable amine which was reacted with 0.5 equivalents of bis(4-nitrophenyl) carbonate, giving protected L-IP3 dimer 16. Hydrogenolytic deprotection of 16 gave L-IP3 dimer 8. When 1 equivalent of bis(4-nitrophenyl) carbonate was used, the product was 4-nitrophenyl N-alkylcarbamate 17, which could be isolated and conjugated with D-IP3 component 11. The conjugation reaction was carried out in CD3OD and monitored by 31P NMR spectroscopy. Deprotection followed by anion-exchange chromatography then gave IP3–L-IP3 hetero-dimer 5. Dimers 6 and 7 were synthesized from alcohol 1849. Nitrile 19 was reduced, and the amine product was temporarily protected as the N-trifluoroacetamide (20). Acid-labile protecting groups were then removed, giving pentaol 21, which was converted, via 22, into carbamate 23. Carbamate 23 was then conjugated with 11, and deprotection followed by anion-exchange chromatography gave IP3–IP5 hetero-dimer 6. Alternatively, conjugation of carbamate 24 with 11 gave IP3–Ins dimer 7. Reagents and conditions: (i) LiOH, THF, MeOH, H2O; (ii) bis(4-nitrophenyl) carbonate (0.5 equiv), THF; (iii) bis(4-nitrophenyl) carbonate (1 equiv), THF; (iv) H2, Pd(OH)2/C, MeOH, H2O; (v) 11, CD3OD, Et3N; (vi) NaH, BrCH2CN, CH3CN; (vii) LiAlH4, THF; (viii) EtOC(O)CF3, THF; (ix) TFA, H2O; (x) (BnO)2PNiPr2, 1H-tetrazole, CH2Cl2 then 3-chloroperoxybenzoic acid; (xi) Et3N, H2O, reflux; (xii) bis(4-nitrophenyl) carbonate (1 equiv), DMF, Et3N. Bn, benzyl; PMB, 4-methoxybenzyl. All experimental procedures are described in detail in Supplementary Methods online.