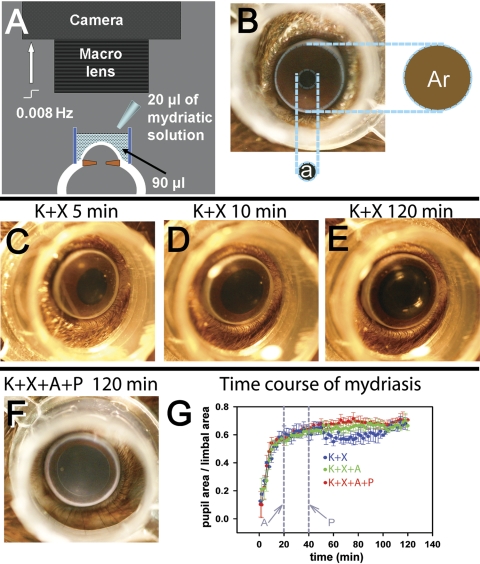
Figure 1.
Measurement of pupillary mydriasis. (A) The setup used for recording pupillary mydriasis. A digital-SLR camera fitted with a macro lens was focused at the pupillary plane of the anesthetized mouse eye. A transparent polypropylene cylinder (outer diameter, 8 mm; height, 5 mm) was centered on the cornea and filled with 90 μL 0.25% sodium carboxymethyl-cellulose solution, to which 20 μL of mydriatic solution was added. The camera was computer controlled to take images at 0.008 Hz. (B) Photograph of a mouse eye showing that pupillary area (a) was expressed as a ratio of the area encircled by the limbus (Ar). (C–E) Representative photographs of a single mouse eye, showing levels of mydriasis 5, 10, and 120 minutes after K+X anesthesia. (F) Representative photograph of a mouse eye, taken 120 minutes after K+X anesthesia and topical A+P administration (topical A, 20 minutes, and topical P, 40 minutes after induction of anesthesia). (G) Time course of average mydriasis expressed as a ratio of the limbal area, produced by K+X anesthesia (n = 4), K+X anesthesia followed by A administered at 20 minutes from induction of anesthesia (n = 3) and K+X anesthesia followed consecutive administration of A at 20 minutes and P at 40 minutes from induction of anesthesia (n = 4). Vertical gray lines: time at which A and P were administered.