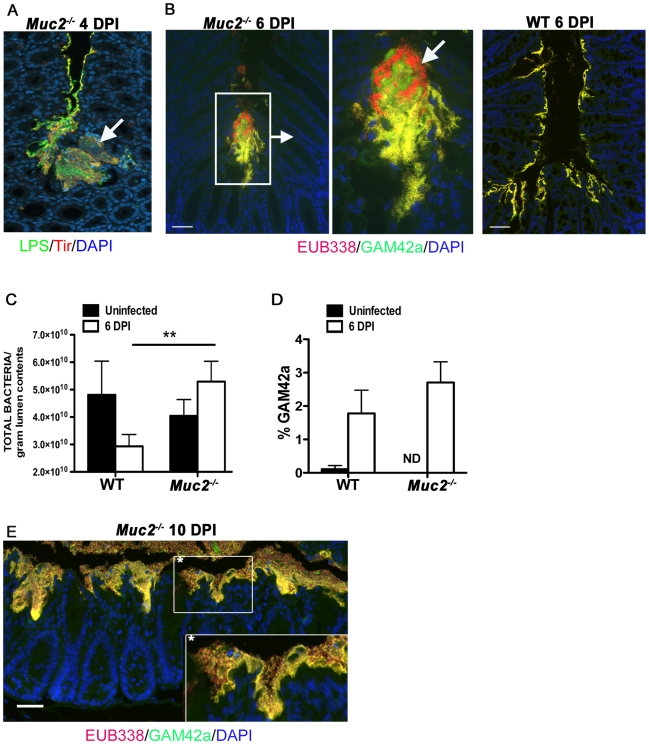
Figure 8. Increased luminal load of both pathogenic and non-pathogenic bacteria in Muc2−/− mice during infection.
A. Immunofluorescence staining for C. rodentium LPS and DAPI in Muc2−/− at 4 DPI Notice DAPI-stained bacteria that are negative for LPS in the C. rodentium microcolonies (arrow). Original magnification = 200×. B. Dual FISH staining using DNA probes that label virtually all true bacteria (EUB338, red) and the γ-Proteobacter class to which C. rodentium belongs (GAM42a, green). Pathogenic bacteria (i.e. EUB338+/GAM42a+ cells) are yellow, and commensal bacteria (EUB338+/GAM42−) cells are red. Note the non-ulcer associated bacterial microcolony containing commensal bacteria (red) mixed in with pathogenic bacteria (yellow) in Muc2−/− mice (left panels). Such mixed microcolonies were not seen in WT mice, which show predominantly pathogenic bacteria intimately adherent to the mucosa (right panel). Tissues were fixed in Carnoy's fixative prior to processing. Original magnification = 200×. Scale bar = 100 µm. C. SYBR green quantification of total bacterial burden per gram of colorectal lumen contents of WT vs. Muc2−/− mice before infection and at 6 DPI. Results are presented as the means of a total of 5–7 mice per group pooled from 2 independent experiments. Error bars = SEM (**P = 0.0082, Mann-Whitney test). D. Graph illustrating the percent composition of γ-Proteobacter (EUB338+/GAM42a+ cells), which is primarily represented by C. rodentium, in colorectal luminal content from uninfected or infected WT vs. Muc2−/− mice. Results are the mean percentages from a total of 5–7 mice per group pooled from 2 independent experiments. ND, none detected. Error bars = SEM. E. FISH staining as described above, showing a thick biofilm of mostly pathogenic but also commensal bacteria on the mucosal surface in a colonic section from a moribund Muc2−/− mouse at 10 DPI (inset). Such phenotypes were not observed in WT mice. Original magnification = 200×. Scale bar = 50 µm.