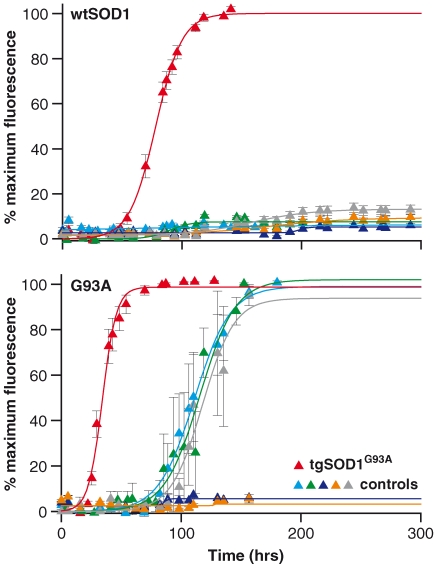
Figure 4. Fibrillogenic seeding with affected tissue homogenates.
wtSOD1 (top graph) and G93A (bottom graph) proteins were seeded with spinal cord homogenates from 120 days old tgSOD1G93A (red triangle), tgSOD1wtSOD1 (light blue triangle) and control non-transgenic littermates of the tgSOD1G93A (green triangle) mice. Seeded reactions spiked with 1% (v/v) tgSOD1G93A spinal cord homogenates into wt SOD1 and G93A proteins shortened the lag times to approximately 56 hrs and 23hrs respectively, comparable to seeded reactions with pre-formed recombinant G93A fibrils to wt SOD1 and to self-protein (G93A) (refer to Table 1). The lag times of seeded reactions with tgSOD1wtSOD1 and non-transgenic control spinal cord homogenates into G93A protein were 1.5–3.5 fold longer than the lag times of seeded reactions by tgSOD1G93A spinal cord homogenates into G93A protein. Seeding with tgSOD1wtSOD1 and non-transgenic control spinal cord homogenates into wtSOD1 protein did not give rise to any ThT fluorescence change, indicating an absence of fibril formation. Additional spinal cord homogenate controls were used to test for seeding specificity as described in the text: spinal cord homogenates were prepared from clinical endstage Tg20 mice infected with the RML strain of prions (dark blue triangle), huntingtin transgenic mice (N171-82Q) (grey triangle), and brain homogenate prepared derived from an FTD3 patient (yellow triangle).Data shown are average values from 4–10 replicates (± SEM) pooled from 2–3 independent assays.