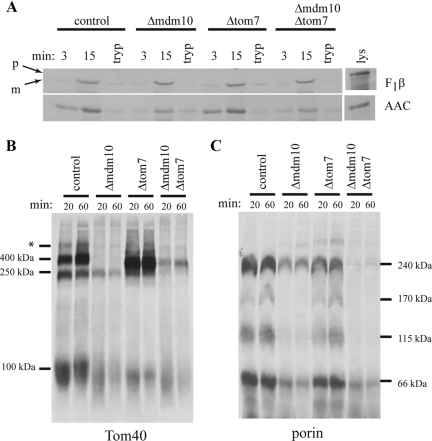
Figure 2.
Import of precursor proteins into mitochondria of mutant strains. (A) Import of radiolabeled matrix-targeted β-subunit of mitochondrial F1 ATP synthase (F1β) and the inner membrane targeted ATP/ADP carrier (AAC) into mitochondria isolated from the indicated strains. After import, the mitochondria were treated with proteinase K, reisolated, electrophoresed, transferred to nitrocellulose membranes, and examined by autoradiography. The time (min) of each import reaction is indicated above the lanes. Lys, 33% of the input lysate containing radiolabeled protein used in each reaction; tryp, mitochondria pretreated with trypsin before 8 min of import with precursor protein. For F1β, arrows indicate the positions of the precursor (p) and mature (m) forms of the protein. (B) Import of radiolabeled Tom40 precursor into mitochondria isolated from the indicated strains. After import, the mitochondria were reisolated and dissolved in 1% digitonin. The samples were subjected to BNGE, transferred to PVDF membrane, and analyzed by autoradiography. The time (min) of each import reaction is indicated above the lanes. The molecular mass of complexes are indicated in kilodaltons. The asterisk (*) indicates a band previously characterized as a nonproductive intermediate (Taylor et al., 2003). (C) As in B, but radiolabeled porin precursor was imported. The molecular mass of the bands detected in B and C are indicated. The faint bands of highest molecular mass in the Δmdm10 and Δtom7 lanes appear in some import experiments but not others and have not been characterized.