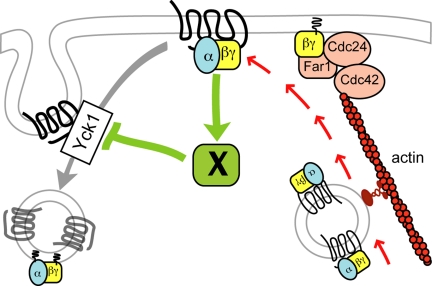
Figure 11.
Induction of receptor polarity by differential receptor internalization. The cartoon illustrates a putative positive feedback loop that accounts for the cell's ability to polarize the pheromone receptor and its G protein before their localized deposition by actin-dependent directed secretion. We postulate that the initially slight gradient of activated receptor and G protein activates and/or recruits an unknown factor (X) that protects ligand-bound receptors from Yck-dependent phosphorylation, thereby inhibiting receptor and G protein internalization. This increases the local concentration of receptor and G protein at the future shmoo site which further stimulates the protective activity of factor X. Eventually, there is a sufficient concentration of Gβγ to define the axis of polarity via recruitment of the Far1–Cdc24–Cdc42 chemotropic complex. This model provides an explanation both for directional sensing in a pheromone gradient and symmetry breaking under isotropic conditions and is consistent with our major findings: 1) Pheromone-induced receptor polarization is absolutely dependent on receptor internalization but can occur in absence of actin-dependent directed secretion. 2) The receptor polarizes before detectable polarization of actin cables and morphogenesis. 3) Polarized receptor crescents are invariably oriented toward potential mating partners when they first appear. 4) Pheromone treatment of cells forced to maintain a uniform distribution of receptor induces hypophosphorylated receptor crescents. We believe Gβγ is a good candidate to be factor X, as we have found that Gβ physically interacts with Yck1 (Stone and Ismael, unpublished data). Note that the coupled delivery of the receptor and G protein to the growth site is purely speculative.