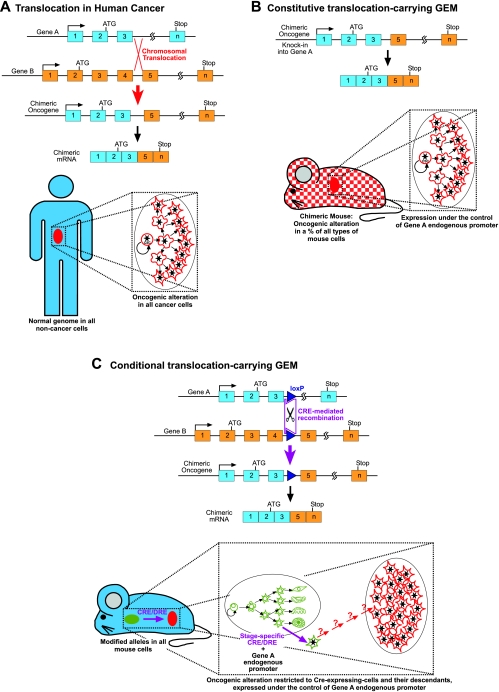
Fig. 2.
Example of the mimicking of a complex human oncogenic alteration in the mouse. (A) Molecular mechanism of a human chromosomal translocation resulting in a chimeric oncogene. (B) Knock-in mouse model of chromosomal translocation. By homologous recombination in ES cells, one allele of gene A is modified to introduce the 3′-elements of gene B in order to mimic the rearrangement seen in humans. These ES cells are injected into wild-type (WT) blastocysts to generate chimeric mice that are composed of WT and genetically modified cells. Therefore, a percentage of the cells in every organ of the chimera carry the oncogenic alteration, which is expressed under the regulatory sequences of gene A, thus generating a model that very closely mimics the human case where tumoral cells are mixed in a background of normal cells. Unfortunately, most of these chimeric mice cannot produce viable knock-in offspring, indicating that fusion proteins are toxic for development. (C) Conditional, Cre-inducible translocation model: genes A and B are modified separately by homologous recombination in ES cells, and loxP sites (or the recently developed Dre-rox sites) are introduced at the precise points where chromosomal translocation happens in humans. F1 mice heterozygous for these modified genes are generated and crossed with a tissue-specific Cre (or Dre) recombinase. These mice carry the modified alleles in all cells and have no phenotype in the absence of recombinase. The oncogene is expressed under the regulatory sequences of gene A in all the cells expressing recombinase and their potential descendants. (The expression of the recombinase under differentiated cell promoters in differentiated cells leads to the appearance of tumors that do not necessarily reproduce the hierarchical structure of human cancers.)