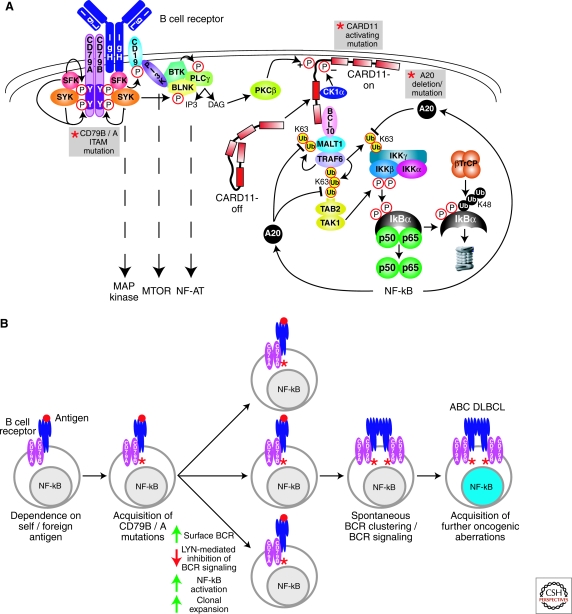
Figure 1.
Role of BCR signaling to NF-κB in ABC DLBCL. (A) Schematic of BCR signaling to NF-κB. Recurrent genetic alterations in ABC DLBCL that result in constitutive NF-κB activation are indicated by the gray boxes. Proximal signaling by the BCR is initiated by SRC-family kinases (SFK; e.g., LYN, FYN, FGR, and BLK), which phosphorylate the ITAM motifs in the CD79A and CD79B components of the BCR receptor. SYK is recruited to the phosphorylated ITAMs and activated to phosphorylate many downstream proteins. The PI(3) kinase pathway is activated by SRC-family kinase phosphorylation of the BCR coreceptor CD19. The generation of PIP3 by PI(3) kinase recruits BTK and associated BLNK and phospholipase Cγ2 (PLCγ2) to the plasma membrane. PLCγ2 generates inositol triphosphate (IP3), which leads to opening of the capacitative calcium channel, thereby activating the NF-AT pathway. Diacylglycerol (DAG) is also generated, which activates protein kinase Cβ (PKCβ). PKCβ phosphorylates the latent form of CARD11 (CARD-off) in the cytoplasm, causing it to adopt an active conformation (CARD11-on), translocate to the plasma membrane, and recruit the signaling adapters BCL10 and MALT1. MALT1 binds TRAF6, causing TRAF6 to catalyze K63-linked polyubiquitination of the IKKγ subunit, MALT1 and itself. TAB2 recognizes the polyubiquitin chains on TRAF6, leading to phosphorylation of IKKβ in its activation loop by TAK1 kinase. Ubiquitination of IKKγ and phosphorylation of IKKβ activate IKKβ to phosphorylate IκBα. Phosphorylated IκBα is ubiquitinated by the ubiquitin ligase βTrCP, leading to its proteasomal degradation. Nuclear NF-κB heterodimers activate multiple target genes, including A20. A20 terminates NF-κB signaling by removing K63-linked ubiquitin chains from IKKγ, TRAF6, and MALT1, and attaching K48-linked ubiquitin chains. (B) The role of chronic active BCR signaling in the pathogenesis of ABC DLBCL. Genetic mutations in CD79B and CD79A ITAM regions in ABC DLBCLs suggest that BCR signaling is key to the pathogenesis of ABC DLBCL. CD79B and CD79A ITAM mutations in the mouse cause hyperactive BCR signaling, suggesting that CD79 ITAM mutations in ABC DLBCL may amplify antigen-stimulated BCR signaling. CD79 ITAM mutations increase surface BCR expression and decrease activation of LYN kinase, a negative regulator of BCR signaling, potentially resulting in increased signaling to NF-κB and greater clonal expansion. A separate step leading to chronic active BCR signaling in ABC DLBCL is the acquisition of spontaneous BCR clustering, which is not caused by the ITAM mutations. The BCR clustering phenotype could theoretically be acquired either before or after the CD79 mutations. Finally, ABC DLBCLs must acquire additional oncogenic hits to become fully malignant.