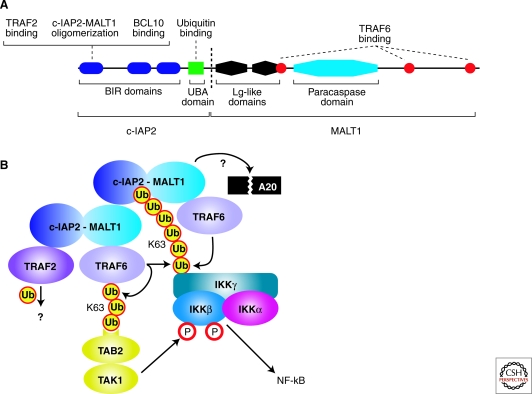
Figure 2.
Pathogenesis of MALT lymphoma. (A) Schematic of the c-IAP2-MALT1 fusion oncoprotein produced by the t (11;18) translocation in MALT lymphomas. Shown is one common fusion breakpoint (dashed line), but other breakpoints occur less frequently (for review, see Isaacson et al. 2004). (B) Molecular mechanisms of NF-κB activation by the c-IAP2-MALT1 fusion protein. c-IAP2-MALT1 forms multimers by heterotypic interactions between its BIR and MALT1 regions. c-IAP2-MALT1 binds TRAF6, activating the K63-linked ubiquitin ligase activity of TRAF6 for IKKγ and itself. Polyubiquitated TRAF6 binds TAB2, thereby activating the associated TAK1 kinase to phosphorylate IKKβ. The ubiquitin-binding domain of c-IAP2-MALT1 stabilizes its interaction with ubiquitinated IKK. The proteolytic cleavage of a substrate protein, potentially A20, by the paracaspase domain of MALT1 is required for the function of c-IAP2-MALT1. TRAF2 is also required for c-IAP2-MALT1 activity, but its substrate is unknown.