SUMMARY
Three physically separate incursions of the raccoon strain of rabies have entered Canada, two into eastern Ontario in 1999 and one into New Brunswick in 2000. The course of these epizootics is described. Phylogenetic analysis of the index cases from these two provinces with raccoon rabies viruses representative of this strain in the United States supported the independence of these incursions into Canada via cross-border transmission from the United States. Genetic characterization of 190 isolates from these two Canadian provinces over a 550-bp region of the variable central portion of the viral P gene distinguished 14 variants in Ontario and five in New Brunswick although in both regions the variant represented by the initial case was most commonly encountered. The quasi-species nature of the Ontario virus was analysed using isolates taken at different times during the main outbreak to examine whether viral variation was increasing with time as well as changing in nature. These data provide a framework for study of future incursions of this rabies strain into Canada.
INTRODUCTION
Despite the fact that the incidence of rabies in the human population of North America has dropped to extremely low levels over the last few decades, averaging, in the United States just 1–2 cases per year [1], this disease continues to be a significant public health problem due to the persistence of the rabies virus in certain wildlife species. Distinct variants of rabies virus, that are to various degrees geographically restricted in range, are associated with specific host vector species which act as reservoirs for the maintenance of the viral variant in both the United States and Canada [2, 3]. In particular, the Canadian province of Ontario reported significant numbers of rabies cases after the introduction of the arctic fox strain from arctic regions into southern Canada in the 1950s [4]. After causing outbreaks throughout eastern Canada in the 1950s and 1960s, this strain disappeared from southern Canada except in certain areas of Ontario where rabies incidence was substantially reduced only when extensive control efforts were initiated in the 1990s [5]. Several other distinct rabies virus variants circulate in certain species of insectivorous bats and such variants are widely dispersed within Canada; indeed in the maritime provinces of New Brunswick, Nova Scotia and Prince Edward Island, infection of terrestrial mammals has for many years been due exclusively to spillover from the chiropteran reservoir [6]. In the United States, the raccoon variant of rabies has become the most problematic rabies strain for a number of reasons. First, since its recognition in the mid-1950s in the state of Florida, it has successfully spread over a very large area, especially following emergence of the mid-Atlantic raccoon strain in West Virginia in the late 1970s, due probably to human-assisted movement of incubating animals into the area from the South. Subsequently this strain spread rapidly, reaching as far north as the state of Maine by 1994. A detailed history of the emergence and spread of the raccoon rabies variant throughout its current range along the entire eastern seaboard of the United States has been described [7–9]. For several years the raccoon (Procyon lotor) has been the species most often reported as rabid in the United States; in 2002 raccoons comprised 36·3% of all cases, with the vast majority of these being due to the raccoon variant [3]. Second, the host vector, the raccoon, adapts very well to urban environments where it may come into close contact with the general human population and their associated domestic animals. Indeed, it has been reported that the incursion of raccoon rabies into an area often results in dramatic increases in expensive human post-exposure prophylaxis [10]. In 2003 the first case of human rabies due to the raccoon variant was recorded in northern Virginia [11]. In light of these observations, significant control efforts have been undertaken to prevent establishment of this rabies variant in areas free of this epizootic but neighbouring those impacted by raccoon rabies. Due to the maintenance of the raccoon rabies strain in neighbouring New York state throughout the 1990s, the province of Ontario has had a raccoon rabies control plan in effect for several years [12] and in 1998 the state of Ohio responded to an epizootic that had entered the eastern part of the state from Pennsylvania by implementing large-scale oral vaccination [13].
In July 1999, the province of Ontario, Canada, reported its first case of raccoon rabies in a small community on the north shore of the St Lawrence River that divides Ontario from New York state [14]. Despite extensive point-control measures that were implemented by the Ontario provincial authorities immediately following this first report [15], raccoon rabies persisted in the province. In September 2000, the raccoon variant of rabies was also reported in the province of New Brunswick in an area bordering with the state of Maine [16]. The introduction of raccoon rabies into two separate regions of Canada provided an opportunity to apply molecular epidemiological techniques to study certain aspects of the introduction and maintenance of these rabies outbreaks.
Rabies virus, the type virus of the Lyssavirus genus, has an enveloped bullet-shaped particle, morphology typical of the Rhabdoviridae family [17]. Seven different genotypes of lyssaviruses are generally recognized with the classical rabies viruses assigned to genotype 1 [18–21]. The rabies virus negative sense single-stranded RNA genome is ∼12 kb in length and is divided into five coding regions designated: 3′-N-P-M-G-L-5′ [22]. Although substantial functional constraints appear to limit the evolution of large portions of the rabies virus genome [23], a pairwise comparison of the nucleotide sequence of rabies virus (PV strain) with a member of the Lyssavirus genotype 3 (Mokola virus) illustrated significant variation in the level of sequence conservation along the length of the genome [24]. Based on this analysis we have previously examined the genetic variation of the P gene locus across the Lyssavirus genus and found that the central region of the P gene is relatively variable [21, 25] and is thus suitable for detailed epidemiological studies [26].
In this report, we describe the targeting of the raccoon rabies variant P gene for a detailed molecular epidemiological study aiming to provide some information on the following issues: (i) to investigate the immediate source of these outbreaks in Canada, and (ii) to study virus variation in the field over time.
METHODS
Viral specimens
All specimens of Canadian origin described in these studies were obtained through the diagnostic facility of the Rabies Centre of Expertise located at Ottawa Laboratory Fallowfield. Specimens were submitted according to the routine measures prescribed for investigation of the rabies status of suspect animals or, in a few instances, as a consequence of the additional surveillance measures that were implemented by provincial authorities in response to the incursion of raccoon rabies into the province of Ontario. For each specimen the date of submission was recorded together with location of origin using a Universal Transverse Mercator Code (UTMC) as described previously [27]. In addition nine specimens of the raccoon strain of rabies, obtained from sources within the United States, were included for comparison. These isolates were: FL125.87R from Florida, MA5142.03R from Massachusetts, NH5154.03R from New Hampshire, NY516.92R, NY8982.98R and NY9581.98S from New York, NJ1927.04E from New Jersey, PA1819.89R from Pennsylvania and VA5231.03R from Virginia. The first two letters of each specimen name indicate state of origin while the final three digits indicate the year of recovery from the field (from 1987 to 2004) and the animal species (E, equine; R, raccoon; S, skunk) of the specimen. The two 1998 New York specimens originated from a region close to the area in Ontario where the index case for that province was found.
Rabies diagnosis and strain typing
Rabies infection was determined in all submitted cases by application of the fluorescent antibody test (FAT) to brain smears [28]. All rabies-positive specimens were further investigated by an indirect FAT applied to additional brain smears using a panel of 16 anti-rabies monoclonal antibodies; the reactivity patterns observed with this panel allowed identification of the strain of the virus responsible for infection. Only those cases demonstrated to be infected with the raccoon strain of rabies were included in this study.
RNA extraction and reverse transcription polymerase chain reaction (RT–PCR)
Tissue of rabies-infected brain (0·1 g) was treated with TRIzol reagent to recover total tissue RNA as directed by the supplier (Invitrogen, Burlington, Canada). Final RNA pellets were dissolved in RNase-free sterile water and RNA concentration determined spectrophotometrically. Amplification of a segment of the rabies virus genome by RT–PCR was achieved essentially as described previously [29]. The rabies virus P gene was targeted with the following primers: RabPfor 5′-CTACTTCTCCGGGGAAACCAGAAG-3′ (corresponding to bases 1249–1272 of positive-sense N gene sequence of the PV strain) was used to prime cDNA synthesis while RabPrev 5′-GGRAGCCAYAGGTCRTCGTCAT-3′ (corresponding to bases 2575–2596 of negative-sense M gene sequence of the PV strain) was used together with RabPfor for the amplification using Taq DNA polymerase (Invitrogen). Thermocycling was performed in a 50 μl reaction on either a 9600 or 9700 system (Applied Biosystems, Foster City, CA, USA); after a 2 min denaturation at 95°C, the cycling conditions were: 95°C, 1 min; 50°C, 1 min; 72°C, 2 min for 30 cycles. Generation of the expected 1348-bp amplicon was confirmed by electrophoresis of an aliquot of each reaction through 1% agarose with visualization of DNA by ethidium bromide staining. In some cases, where insufficient product was generated after a single round of PCR, a second round of amplification was performed using a 2-μl aliquot of the first-round reaction and internal primers Pseqfor 5′-GAGATGGCAGAGGARACTGTAGATCT-3′ (corresponding to bases 1568–1593 of the PV strain) and Pseqrev 5′-CCTTAACTATGTCRTCAAGRTTCA-3′ (corresponding to bases 2208–2231 of the PV strain); the size of the resulting amplicon was 664 bp. Cycling conditions similar to those used for the first-round PCR were applied except that the 72°C extension was only for 1·5 min and 35 cycles were employed. PCR products were purified using a Wizard PCR Preps Purification system (Promega, Madison, WI, USA) prior to consensus nucleotide sequencing.
Nucleotide sequencing and data analysis
Initially, sequencing was performed manually using 32P-labelled sequencing primers (Pseqfor/Pseqrev) and a fmol cycle sequencing kit according to the manufacturer’s (Promega) specifications on a model SA sequencing system (Gibco BRL, Gaithersburg, MD, USA). More recently, a 4200L two dye automated sequencing system (Li-Cor, Lincoln, NE, USA) was used together with custom IR 700 and IR 800 labelled primers (Li-Cor) and a Thermosequenase cycle sequencing kit (Amersham Biosciences, Baie d’Urfé, PQ, Canada). Nucleotide sequences representative of all raccoon rabies strain variants generated during this study have been deposited in GenBank and assigned the following accession numbers: AY854603 to AY854611 for nine complete P gene sequences, AY854612 to AY854625 for ONT1 – 14 variant partial P gene sequences, AY854626 to AY854630 for NB1–5 variant partial P gene sequences. Two complete P gene sequences, for isolates NY516.92R and FL125.87R, were previously deposited and have accession numbers AF369293-4.
The DNASIS® package (Helixx Technologies, Inc., Scarborough, ON, Canada) was used to translate nucleotide sequences to protein sequences.
Alignments were generated using the ClustalX 1.8 software [30] and displayed in phylip format using the dnapars and protpars programs of the phylip 3.5 software package [31]. All phylogenetic analysis was performed using the phylip 3.5 software package of programs. Bootstrap replicates of the nucleotide sequence data were generated using seqboot and the dnadist program was used to determine distance values for these data. Neighbour-joining (NJ) analysis of the distance data was achieved with the neighbor and consense programs; fitch was used to reapply distance values to the consensus tree and the treeview program [32] was used to generate a graphical output of the tree. Tree generation was also performed by DNA parsimony methods using the dnapars program of phylip.
Quasi-species analysis of selected isolates
Three Ontario specimens were selected for this analysis: the second reported case in 1999 and two cases from May 2003 which were among the last specimens included in the study and which corresponded to variants ONT8 and ONT9. All three original RNA preparations were reverse transcribed and then amplified using the ExpandTM high-fidelity plus amplification system (Roche Applied Science, Laval, PQ, Canada) which includes Taq DNA polymerase and a proof-reading enzyme that significantly reduces amplification-induced errors. The resulting PCR products were directly sequenced to confirm the prior variant designation and then subjected to TA cloning into the pCR1.2 vector (Invitrogen). Twenty molecular clones of each PCR product were recovered and sequenced using the automated sequencing protocol as described. These 20 sequences were aligned, using the PCR consensus sequence as reference, using ClustalX 1.8. Statistical analysis of total mutations observed for each isolate was undertaken using SigmaSTAT software version 2.03 (SPSS Inc., Chicago, IL, USA).
Mapping of raccoon rabies cases
Maps were completed on ArcGis 8.3 using the spatial analyst extension. The UTMC value collected with each raccoon rabies case was converted to a set of x,y-coordinates and added to the project using the NAD 83 projection system. Provincial data, created from a geodatabase, was added to the project to aid in identifying borders and clarifying point-specific locations. Using Spatial Analyst (ArcGis extension) case density was calculated using a kernel density configuration; a search radius of 2500 m at a resolution of 30 was employed around selected points. Once complete, the number of classes created was reduced from a value of 9 to 5 with exclusion of the zero value. To determine the value or density within these ranges, a calculation was made to determine the number of points that fell within this value. Finally the grey scale was determined by using a lighter grey for lower density to full black for high density and significant geographical features were labelled. Completed maps were exported in a .jpg format.
RESULTS
Case surveillance summary
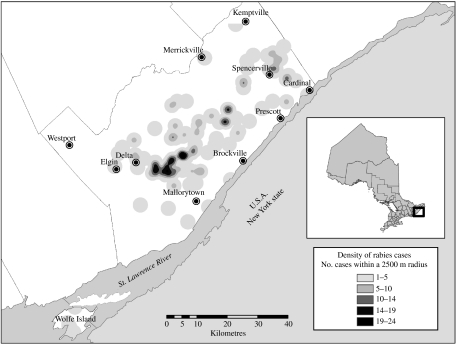
The two areas affected by the raccoon strain of rabies are illustrated in Figures 1 and 2. The first case of raccoon rabies identified in eastern Ontario (Fig. 1) and reported on 14 July 1999, was recovered near the town of Prescott on the north shore of the St Lawrence seaway very close to a bridge linking Ontario to New York state. A second case recovered ∼15 km west of the index case was reported on 26 July 1999 and the third, not identified until 17 September, came from an area ∼15 km north of the initial report. A second outbreak, physically separated by ∼90 km from the first, occurred on Wolfe Island located at the eastern end of Lake Ontario where it drains into the St Lawrence River; of note is the fact that Wolfe Island is serviced by ferries operating from both Ontario and New York. A total of six raccoons, taken from Wolfe Island between 10 December 1999 and 18 January 2000, were confirmed rabid by laboratory testing, but no subsequent cases have been reported. Despite control efforts directed to the first outbreak, this epizootic persisted; while there was very little movement of the outbreak eastwards the epizootic very slowly spread both to the west, south-west and north from the area reporting the index case. By July 2001, the outbreak reached Elgin, ∼55 km west of the index case and in May 2003 the town of Kemptville, ∼30 km north of the index case. An interim description of the geographical distribution of this epizootic has appeared in print [33].
Fig. 1.
Map of the Ontario raccoon rabies epizootic area showing density of cases reported over the study period. The inset map of the province of Ontario identifies the region of eastern Ontario affected by raccoon rabies. The density of raccoon rabies cases is illustrated in the main map as a grey scale using the density scale at bottom right. Locations of communities mentioned in the text are also shown.
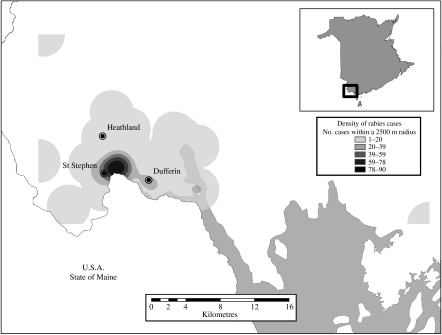
Fig. 2.
Map of the New Brunswick raccoon rabies epizootic area showing density of cases reported over the study period as for Figure 1. The inset map of New Brunswick province identifies the affected region shown in the main map.
The first case of raccoon strain rabies in New Brunswick (Fig. 2), reported on 12 September 2000, was retrieved from the town of Heathland in Charlotte county which lies on the US border with Maine [16]. Between the autumn of 2000 and May 2002 many additional cases were reported from this same area; the epizootic appeared to be confined to St Stephen, the major town of the area which is in close proximity to a bridge connecting Canada with the United States, and neighbouring communities within ∼20 km radius of St Stephen.
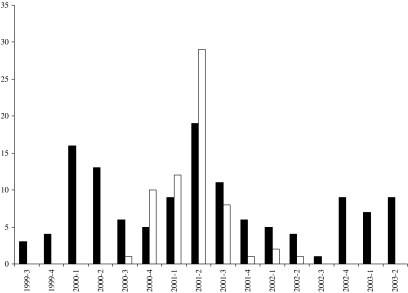
Figure 3 summarizes, according to province, all reported cases of the raccoon strain of rabies in Canada on a quarterly schedule from the first case in Ontario in July 1999, up to the end of 2003. Of the 191 total cases, 127 were reported in Ontario and 64 in New Brunswick. All but 11 cases were reported in raccoons with the striped skunk being the only other species infected with this strain of rabies, two in Ontario and nine in New Brunswick. Indeed the New Brunswick outbreak was notable in that the first three reported cases of raccoon rabies occurred in skunks and, moreover, of the first 16 cases that were recorded in this province from September 2000 to the end of January 2001 the skunk was the affected species for 50% of the specimens; thereafter raccoons became the predominant species affected. Whereas the outbreak in New Brunswick describes a single peak reaching a maximum (29 cases) in the second quarter of 2001 followed by a decline over the following year, the pattern in Ontario is more irregular and exhibits several peaks and troughs without apparently following any clear seasonal pattern. No cases of raccoon strain rabies were reported in either province in the final two quarters of 2003.
Fig. 3.
Quarterly number of raccoon strain rabies cases in Ontario (▪) and New Brunswick (□).
Genetic characterization of the index viruses from Ontario and New Brunswick
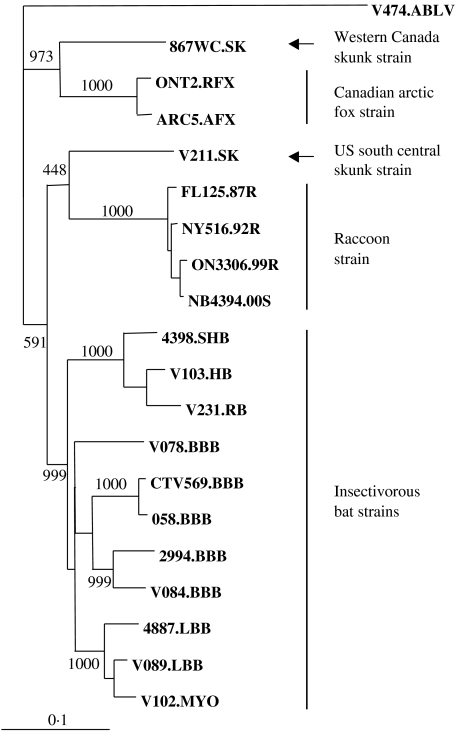
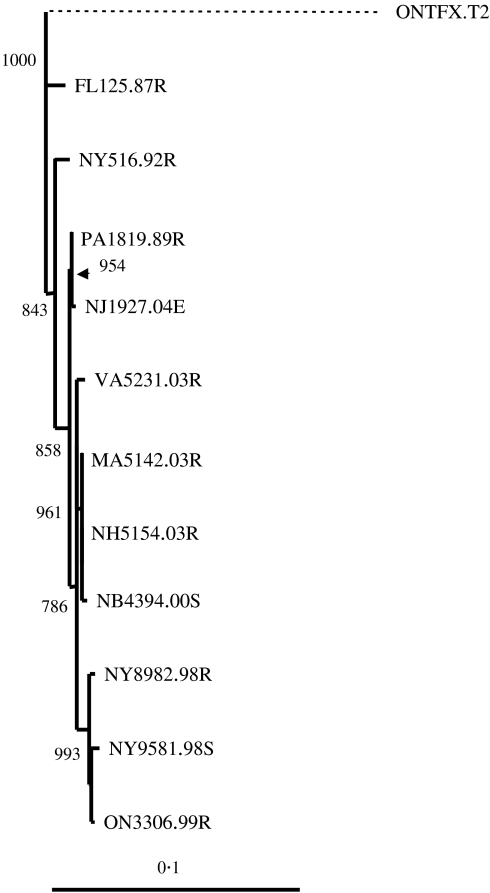
First, to confirm the designation of the two viruses first recovered from Ontario and New Brunswick as the raccoon rabies strain, as indicated by antigenic characterization, the complete coding region of the viral P gene has been sequenced in each case and compared phylogenetically to 17 rabies viruses representative of the major viral strains known to circulate in North America. The pairwise distance values thus determined (data not shown) clearly indicated a clustering of the New Brunswick and Ontario isolates with the Florida and New York raccoon strain isolates (distance <0·03) compared to all other isolates (distance >0·19). A NJ tree, shown in Figure 4, clearly illustrates the close relationship of these four isolates and their distinctiveness from all other isolates representing various bat strains and strains of other terrestrial species, including the arctic fox, the western Canada skunk and the US south central skunk variants.
Fig. 4.
Phylogeny of 19 rabies viruses, and an Australian bat lyssavirus isolate used as an outgroup, using a NJ algorithm applied to complete P gene coding sequences. The two raccoon rabies isolates first recovered in the provinces of Ontario and New Brunswick were compared to 17 other rabies viruses representative of the main variants circulating in the United States and Canada. Apart from the sequence of an isolate recovered from a big brown bat in Connecticut (CTV569.BBB), the P gene sequences of these additional isolates have been described previously [21]. Bootstrap values out of 1000 replicates are indicated for major branches and the distance scale is indicated at bottom left.
To further investigate the origins of these two Canadian rabies viruses, they were compared at the P gene locus to a total of nine raccoon strain rabies viruses recovered from throughout the range of the virus within the United States (see Methods section). The greatest nucleotide distance (0·0284) was observed between the Florida sample and a recent New York isolate (NY9581.98S) although both NY8982.98R and the Ontario sample were almost as divergent from the Florida specimen with values close to 0·0264. The two most closely related samples were the MA5142.03R and NH5154.03R which were identical (distance of 0); the ON3306.99R and NY8982.98R samples differed only slightly (distance of 0·00386) whilst the ON3306.99R and NB4394.00S specimens yielded an intermediate distance value of 0·011638.
These relationships are illustrated in Figure 5 which shows the results of a NJ phylogenetic analysis. With the exception of the placement of the New York sample from 1992, the genetic distances represented by this tree tend to parallel the geographical flow of virus from the south (Florida) to the North (Ontario). Moreover, the close genetic association of the Ontario specimen with 1998 isolates from New York (bootstrap value for cluster is 993) is consistent with the commonly held belief that the Ontario outbreak was due to incursion across the border from this northernmost state. Notably the Ontario and New Brunswick viruses do not cluster together, an observation that supports the independent incursion of these two viruses into Canada; rather the New Brunswick isolate clustered with high confidence with the Masachussetts and New Hampshire isolates (bootstrap value of 961). Additional phylogenetic studies using a maximum parsimony algorithm generated a tree (data not shown) with rather similar topology except that the Virginia sample, the position of which was not supported by high bootstrap values in either tree, clustered with the Ontario/New York group (bootstrap value of 500) rather than with the New Brunswick/New Hampshire/Masachussetts group. In both trees the clustering of the two 1998 New York isolates with the Ontario isolate was highly supported (bootstrap value of  98%) as was the clustering of the New Brunswick isolate with the Masachussetts/New Hampshire samples (bootstrap value of
98%) as was the clustering of the New Brunswick isolate with the Masachussetts/New Hampshire samples (bootstrap value of  90%).
90%).
Fig. 5.
Phylogeny of 11 raccoon rabies viruses as determined using their complete P gene coding sequences with a NJ algorithm and the dnadist program. Bootstrap values out of 1000 replicates are indicated for most branches. An isolate of the arctic fox strain was used as an outgroup and its distance with respect to the raccoon viruses has been reduced (dashed line) to better illustrate the branching patterns of the raccoon isolates.
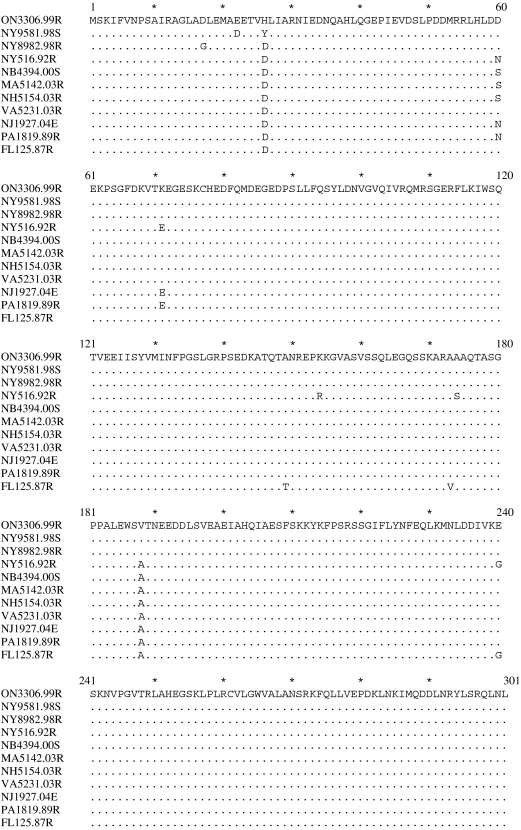
At the amino-acid level distance values were often as great as for nucleotide sequence data and curiously the two most divergent sequences were between NY516.92R and NY9581.98S (0·02708), although overall the Florida sample was again the most divergent with distance values in the range of 0·010–0·020 against all other isolates. Viruses NB4394.00S, MA5142.03R and NH5154.03R all encoded identi-cal P proteins as did isolates NJ1927.04E and PA1819.89R. The 1998 New York and Ontario specimens exhibited minimal differences (distance of 0·006676). A comparison of P protein sequences predicted for all 11 viruses is shown in Figure 6. In all cases the predicted P gene product was a protein of 301 amino acids, four residues longer than the phosphoprotein determined for most rabies viruses [21], indicating that this extended open reading frame is a common feature of the raccoon strain.
Fig. 6.
Alignment of the predicted P proteins of 11 raccoon rabies viruses using the sequence of the Ontario index case as reference. Dots represent identity with the reference sequence.
Variation in the raccoon strain virus
The availability of tissues from almost all of the cases of raccoon rabies that have been reported in Canada to date provided us with the opportunity to explore patterns of virus mutation from both a temporal and geographical perspective. Of the 191 reported cases, amplification of either the complete or a partial viral P gene was successfully achieved for 190 specimens. The amplicons thus generated were sequenced over a 550-bp segment of P gene (corresponding to bases 1616–2165 of the PV reference rabies strain) and variation within this region was scored. The sequences representing the two index viruses in Ontario and New Brunswick were assigned the designation ONT1 and NB1 respectively. Any sequences exhibiting one or more clear nucleotide differences from these sequences were assigned a new numbered designation, e.g. ONT2, -3, etc., and all 190 virus specimens were assigned to a specific sequence variant. The Table summarizes the number of viral specimens corresponding to each variant sequence that was recovered, the range of dates during which that variant was recovered from the field and the nature of the difference from the reference sequence (ONT1 or NB1). It should be noted that to combine our earlier sequence data obtained by manual sequencing, which generated a shorter read length, with the later data generated by an automated system, re-sequencing of a total of 87 PCR products was required. Out of this group, 15 samples had to be re-amplified thereby allowing direct comparison of the sequence obtained from two independent PCRs over a window of ∼400 bp per sample. No discordant sequence was found within these 6000 bases, an observation supporting the relatively high fidelity observed for the consensus sequence analysis.
Table.
Summary of raccoon rabies virus variants
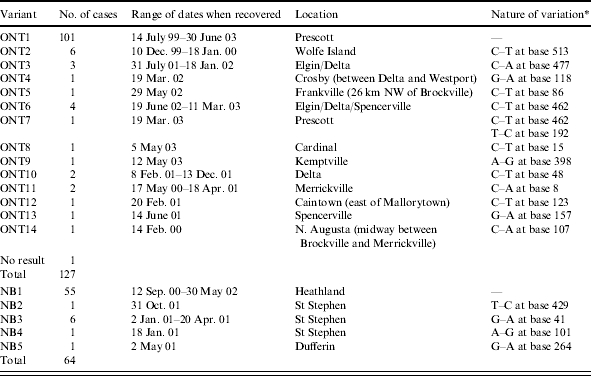
Differences from ONT1 are indicated for the Ontario variants and differences from NB1 are indicated for the New Brunswick samples.
The sequence data described in the Table clearly demonstrate the very limited variation that was observed between all viral samples. In Ontario, although a total of 14 variant sequences were observed over the sequence window studied, most of these varied by just one nucleotide substitution from the index case represented by ONT1. The exception was variant ONT7 which varied by two bases from ONT1, at base 462 similarly to ONT6 and at base 192. Moreover with the exception of variants ONT2, -3, -6, -10 and -11 only a single isolate representative of each variant was observed. For each variant that was identified more than once, its observed geographical and temporal range was quite variable. The ONT2 variant was recovered exclusively from Wolfe Island, an area physically separated from the main Ontario outbreak and all isolates of this type were recovered within a 6-week period (December 1999–January 2000). ONT3 variants originated from two locations ∼8 km apart: Elgin (two isolates recovered with a 6-month separation) and Delta (one isolate obtained just over a week after the first case in Elgin). ONT6 also originated from these same towns (three isolates over a 3-month period) as well as from Spencerville (one isolate) a town located at the other end of the epizootic area 9 months after the first ONT6 case was recovered. ONT10 was recovered from Delta whilst ONT11 came from Merrickville and Mallorytown. With the exception of three C–A changes, all substitutions were transitions. Translation of all 14 of these ONT variant sequences indicated that of the total of 13 base positions that exhibit changes, 6 are synonymous third-base changes (bases 15, 48, 123, 192, 462, 513), one is a non-synonymous third-base change (base 477), four are second-base non-synonymous changes (bases 8, 86, 107 and 398) and two first-base changes at bases 118 and 157 are also non-synonymous. Over the 550-bp sequence window the NB1 variant exhibited six base substitutions compared to ONT1 – it was noted additionally that in all of the viruses that were sequenced through the region flanking the start of this sequence that the NB1 variant also differed by an A–C change seven bases prior to base 1. Four additional variants (NB2–NB5) were observed from the affected area of New Brunswick, each differing by a single base substitution (all transitions) from NB1. NB2, found in a single isolate recovered just over a year after the initial incursion, exhibited a single third-base synonymous substitution at base 429; NB3 variants (six isolates recovered over a 4-month period) exhibited a non-synonymous second-base substitution at base 41; the single NB4 variant exhibited a non-synonymous second-base substitution at base 101 and variant NB5 exhibited a synonymous third-base difference at base 264. These data show that in both study areas the variant that was initially recovered was the one that circulated by far the most widely and was still circulating at the end of the study period.
Quasi-species analysis of selected isolates
To explore the inherent variation at the P gene locus within individual isolates of the raccoon rabies virus three Ontario specimens were analysed further. The specimens chosen for this study included specimen 99RABN03545 recovered in 1999 at the start of the outbreak and two others, specimens 03RABN03406 (ONT8 variant) and 03RABN03574 (ONT9 variant) collected 46 months after the index case. These specimens were re-amplified at the rabies P locus using a proof-reading amplification enzyme mixture (see the Methods section). Twenty molecular clones of each resulting PCR product were recovered and sequenced over an 800-base window starting at base 1628 of the PV reference strain. Specimen 99RABN03545 yielded a total of seven mutations in the 20 cloned sequences corresponding to 0, 3 and 4 changes at the three (1,2,3) codon positions respectively and resulting in three synonymous changes and four non-synonymous differences; all but one of these mutations occurred within the first 400 bases of the sequence window. For both of the 2003 specimens, the consensus sequence of the newly generated PCR product confirmed the variant designation assigned previously, thereby supporting the conclusion that these variations were indeed due to viral variation rather than mutation introduced by the amplification process. All 20 clones generated from the ONT8 variant specimen carried the mutant that determined the variant designation with 12 other mutations observed (3 at the first codon position, 4 at the second and 5 at the third), thereby conferring four synonymous and eight non-synonymous changes. The transition:transversion ratio (Tn:Tv) was 11:1. Eighteen mutations from the consensus sequence were observed in the clones generated from the ONT9 specimen of which five were at the variant-specific base and represented the original ONT1 variant sequence; thus, this specimen was a mixture of the original ONT1 type and the new ONT9 variant. The remaining 13 mutations (Tn:Tv ratio of 12:1) were divided into three at the first-base position, seven at the second base and three at the third base; only three of all these mutations were synonymous. To examine the statistical significance of these mutation levels, mutation scores for all 20 clones of each isolate were subject to analysis by a Mann–Whitney rank sum test. This test indicated that the differences in the median for mutations observed either for the ONT1 and ONT8 or the ONT1 and ONT9 clones were not statistically significant (P=0·606 and 0·315 respectively).
DISCUSSION
While it has generally been assumed that the entry of raccoon rabies into two provinces, Ontario and New Brunswick, in different regions of Canada were independent events, the data in this report clearly substantiate this claim for the first time. Genetic variant analysis clearly discriminates the viruses circulating in these two areas and predicts their association with distinct lineages of this rabies virus strain. Thus, it can be concluded that these two major rabies outbreaks were the result of two instances of cross-border transmission rather than a single incursion into Ontario followed by iatrogenic movement of animals into New Brunswick. Indeed, a third well-contained outbreak on Wolfe Island on the Ontario side of the St Lawrence River probably constituted a third incursion due to its physical separation from the mainland foci and its genetic distinctness; all isolates from this outbreak were of variant ONT2.
The data presented here for the mainland Ontario outbreak are consistent with the introduction of a single viral variant which persisted and spread, with occasional point mutations arising periodically to generate new viral variants. However, these new variants did not generally persist even when the newly introduced mutation was apparently neutral in nature, so that even after several years in the field the genetic variant that was originally introduced into the area still predominated. The second Ontario incursion was short-lived, possibly due to the control efforts that were instigated on the island. In New Brunswick the predominance of one single variant again suggested that this outbreak was due primarily to a single incursion event with maintenance of that viral progeny over the study period, although without information on the nature of the virus circulating in Maine at that time we cannot exclude the possibility that the group of six NB3 variants reported over a period of almost 4 months in 2001 were not due to a second incursion.
The relative genetic stability of these viruses is notable given that one of the most divergent coding regions of the virus was targeted for these analyses. Indeed, based on data published by LeMercier et al. [24] the only viral region that may be more sensitive to genetic change is that encoding the transmembrane and intracellular domains of the glycoprotein as well as the neighbouring non-coding G–L intergenic region. However, genetic characterization of several isolates of this rabies virus, recovered from throughout its range within the United States, corroborated these findings. Variation of this strain is very limited, an observation that may reflect the fact that this strain has been recognized only in relatively recent times (since the mid-1940s in Florida) and may be a newly emerged virus/host association as a consequence of spillover from another rabies reservoir [20]; such an event undoubtedly would represent a significant bottleneck event. Further, due to the extensive efforts undertaken by provincial wildlife authorities to minimize the possible entry and persistence of raccoon rabies in Ontario, the Ontario virus variant underwent another severe bottleneck event which may again have contributed to the very low genetic variation observed.
A review of the nature of the mutations observed in these studies is of interest since it was reported that synonymous base changes tend to predominate due to constraints that operate on rabies virus G and N genes in nature [23]. Of all the 17 mutations observed from our consensus sequence analysis from viruses of both provinces (13 in Ontario and four in New Brunswick), eight were synonymous and nine were non-synonymous; there was thus an approximately equal number of both mutation types and while there was a tendency to find synonymous mutations more frequently (in more isolates) there did not appear to be a very strong correlation in this regard as non-synonymous mutations were also observed multiple times. This rather high proportion of non-synonymous mutations appears to be a feature of this particular region of the P gene [21] indicating that this coding region is under relatively relaxed constraints compared to other regions of the rabies virus genome. The Tn:Tv ratio for all viral variant consensus sequences was 5:1; this ratio may reflect both the relative ease of occurrence of transition mutations as well as selective pressures that may operate against transversions that are more likely to yield non-synonymous changes; indeed all three transversions noted in the variant consensus sequences resulted in a coding change.
Similar trends were evident from the quasi-species analysis performed on three Ontario isolates. For specimen 99RABN03545 four of seven mutations observed were non-synonymous and the Tn:Tv ratio was 4:3. In the isolates recovered in 2003 the proportion of non-synonymous mutations was even higher; eight out of 12 for 03RABN03406 (Tn:Tv ratio of 11:1) and 10 out of 13 (Tn:Tv ratio of 12:1) for 03RABN03574, even when excluding mutations at the site of the original variant change. While we cannot rule out the possibility that some of the mutations observed during the quasi-species analysis could have been introduced by the RT–PCR amplification process, despite the use of a proof-reading amplification system, it appeared that more variation was evident within the 2003 isolates compared to the 1999 specimen, although this was not scored as statistically significant. A longer term study would be required to fully establish this point.
At the level of the phosphoprotein, except for a small number of substitutions, especially at residues 60, 71 and 149–190, regions of the protein identified previously as being highly divergent [21], again the raccoon strain P appears highly conserved throughout its range (see Fig. 6). No variation is evident at positions believed to be functionally important, including conserved methionine residues at positions 20, 53 and 83, which may direct internal translation initiation [34], as well as the LC8 dynein light-chain-binding domain at residues 143–148 [35]. Four of five serine residues (positions 63, 162, 210 and 271) identified as phosphorylation targets in the CVS strain [36] are also consistently retained.
Provincial control efforts aimed at eradicating raccoon rabies were implemented in both provinces [15, 37]. The extent to which these measures were effective in eliminating these outbreaks is unclear, although it is notable that no case of raccoon rabies has been reported in New Brunswick since May 2002, and Ontario reported no cases from June 2003 up to June 2004. The genetic database generated during these studies will be helpful to evaluate any further cases of raccoon rabies that occur in Canada since it may permit discrimination between renewed activity of these former outbreaks or re-incursion from the United States.
ACKNOWLEDGEMENTS
We thank the following investigators for providing the US isolates: NY516.92R, NY8982.98R and NY9581.98S were a gift from C. Trimarchi and R. Rudd of the Rabies Laboratory, New York State Health Dept in Albany, NY; PA1819.89R from Pennsylvania was a gift from C. Rupprecht now of the Centers for Disease Control and Prevention in Atlanta, Georgia; specimens MA5142.03R from Massachusetts, NH5154.03R from New Hampshire, NJ1927.04E from New Jersey and VA5231.03R from Virginia were provided by L. Orciari of the Centers for Disease Control and Prevention in Atlanta, Georgia; FL125.87R was from W. Bigler of Florida. We acknowledge the Rabies programme of the Ontario Ministry of Natural Resources for its financial support of this work. We also thank Sheona Craig, Mary Sheen, Geoff Turner and Marcie Campbell for excellent technical assistance and Bruce Craig for generating the maps shown in Figures 1 and 2.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Messenger SL, Smith JS, Rupprecht CE. Emerging epidemiology of bat-associated cryptic cases of rabies in humans in the United States. Emerg Infect. 2002;35:738–747. doi: 10.1086/342387. [DOI] [PubMed] [Google Scholar]
- 2.Smith JS, Orciari LA, Yager PA. Molecular Epidemiology of rabies in the United States. Sem Virol. 1995;6:387–400. [Google Scholar]
- 3.Krebs JW, Wheeling JT, Childs JE. Rabies surveillance in the United States during 2002. J Am Vet Med Assoc. 2003;223:1736–1748. doi: 10.2460/javma.2003.223.1736. [DOI] [PubMed] [Google Scholar]
- 4.Tabel H, Corner AH, Webster WA, Casey CA. History and epizootiology of rabies in Canada. Can Vet J. 1974;15:271–281. [PMC free article] [PubMed] [Google Scholar]
- 5.MacInnes CD, Smith SM, Tinline RR et al. Elimination of rabies from red foxes in Eastern Ontario. J Wildl Dis. 2001;37:119–132. doi: 10.7589/0090-3558-37.1.119. [DOI] [PubMed] [Google Scholar]
- 6.Nadin-Davis SA, Huang W, Armstrong J et al. Antigenic and genetic divergence of rabies virus from bat species indigenous to Canada. Virus Res. 2001;74:139–156. doi: 10.1016/s0168-1702(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 7.Winkler WG, Jenkins SR, Baer GM. The natural history of rabies. 2nd edn. Boca Raton: CRC Press; 1991. Raccoon rabies; pp. 325–340. [Google Scholar]
- 8.Cartter ML, Cooper GH, Hadler JL et al. Raccoon rabies epizootic – United States, 1993. Morb Mortal Wkly Rep. 1994;43:269–273. [PubMed] [Google Scholar]
- 9.Wandeler AI, Rosatte RC, Williams D et al. Update: Raccoon rabies epizootic – United States and Canada, 1999. Morb Mortal Wkly Rep. 2000;49:31–35. [PubMed] [Google Scholar]
- 10.CDC. Update: raccoon rabies epizootic – United States, 1996. Morb Mortal Wkly Rep. 1997;45:1116–1120. [PubMed] [Google Scholar]
- 11.Silverstein MA, Selgado CD, Bassin S et al. First human death associated with raccoon rabies – Virginia, 2003. Morb Mortal Wkly Rep. 2004;52:1102–1103. [PubMed] [Google Scholar]
- 12.Rosatte RC, MacInnes CD, Taylor-Williams R, Williams O. A proactive prevention strategy for raccoon rabies in Ontario, Canada. Wildl Soc Bull. 1997;25:110–116. [Google Scholar]
- 13.Krebs JW, Smith JS, Rupprecht CE, Childs JE. Rabies surveillance in the United States during 1998. J Am Vet Med Assoc. 1999;215:1786–1798. [PubMed] [Google Scholar]
- 14.Wandeler AI, Salsberg E. Raccoon rabies in eastern Ontario. Can Vet J. 1999;40:731. [PMC free article] [PubMed] [Google Scholar]
- 15.Rosatte RC, Donovan D, Allan M et al. Emergency response to raccoon rabies introduction into Ontario. J Wildl Dis. 2001;37:265–279. doi: 10.7589/0090-3558-37.2.265. [DOI] [PubMed] [Google Scholar]
- 16.MacInnes CD. Raccoon rabies in New Brunswick. The Rabies Reporter. 2000;11:10. [Google Scholar]
- 17.Tordo N, Charlton K, Wandeler A, Collier LH. Topley and Wilson’s microbiology and microbial infections. London: Arnold Press; 1998. Rhabdoviruses: Rabies; pp. 666–692. [Google Scholar]
- 18.Bourhy H, Bachir K, Tordo N. Molecular diversity of the Lyssavirus genus. Virology. 1993;194:70–81. doi: 10.1006/viro.1993.1236. [DOI] [PubMed] [Google Scholar]
- 19.Gould AR, Hyatt AD, Lunt R, Kattenbelt JA, Hengstberger S, Blacksell SD. Characterisation of a novel lyssavirus isolated from Pteropid bats in Australia. Virus Res. 1998;54:165–187. doi: 10.1016/s0168-1702(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 20.Badrane H, Tordo N. Host-switching in Lyssavirus history from the chiroptera to the carnivora orders. J Virol. 2001;75:8096–8104. doi: 10.1128/JVI.75.17.8096-8104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadin-Davis SA, Abdel-Malik M, Armstrong J, Wandeler AI. Lyssavirus P gene characterisation provides insights into the phylogeny of the genus and identifies structural similarities and diversity within the encoded phosphoprotein. Virology. 2002;298:286–305. doi: 10.1006/viro.2002.1492. [DOI] [PubMed] [Google Scholar]
- 22.Tordo N, Meslin FX, Kaplan MM, Koprowski H. Laboratory techniques in rabies. 4th edn. Geneva: WHO; 1996. Characteristics and molecular biology of the rabies virus; pp. 28–51. [Google Scholar]
- 23.Holmes EC, Woelk CH, Kassis R, Bourhy H. Genetic constraints and the adaptive evolution of rabies virus in nature. Virology. 2002;292:247–257. doi: 10.1006/viro.2001.1271. [DOI] [PubMed] [Google Scholar]
- 24.Le Mercier P, Jacob Y, Tordo N. The complete Mokola virus genome sequence: structure of the RNA-dependent RNA polymerase. J Gen Virol. 1997;78:1571–1576. doi: 10.1099/0022-1317-78-7-1571. [DOI] [PubMed] [Google Scholar]
- 25.Nadin-Davis SA, Huang W, Wandeler AI. Polymorphism of rabies viruses within the phosphoprotein and matrix protein genes. Arch Virol. 1997;142:1–14. doi: 10.1007/s007050050133. [DOI] [PubMed] [Google Scholar]
- 26.Nadin-Davis SA, Simani S, Armstrong J, Fayaz A, Wandeler AI. Molecular and antigenic characterization of rabies viruses from Iran identifies variants with distinct epidemiological origins. Epidemiol Infect. 2003;131:777–790. doi: 10.1017/s095026880300863x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tinline R, Gregory D. The Universal Transverse Mercator Code: a location code for disease mapping. Can Vet J. 1984;29:825–829. [PMC free article] [PubMed] [Google Scholar]
- 28.Dean DJ, Ableseth MK, Atanasiu P, Meslin FX, Kaplan MM, Koprowski H. Laboratory techniques in rabies. 4th edn. Geneva: WHO; 1996. The fluorescent antibody test; pp. 88–95. [Google Scholar]
- 29.Nadin-Davis SA. Polymerase chain reaction protocols for rabies virus discrimination. J Virol Meth. 1998;75:1–8. doi: 10.1016/s0166-0934(98)00106-2. [DOI] [PubMed] [Google Scholar]
- 30.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felsenstein J. PHYLIP: Phylogeny inference package, version 3.52c. University of Washington; Seattle, WA: 1993. [Google Scholar]
- 32.Page RDM. TREEVIEW: An application to display phylogenetic trees on personal computers. Comp Appl Bioscience. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 33.MacInnes CD, Stevenson B. Rabies in Ontario – second quarter of 2001. The Rabies Reporter. 2001;12:1–3. [Google Scholar]
- 34.Chenik M, Chebli K, Blondel D. Translation initiation at alternate in-frame AUG codons in the rabies virus phosphoprotein mRNA is mediated by a ribosomal leaky scanning mechanism. J Virol. 1995;69:707–712. doi: 10.1128/jvi.69.2.707-712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poisson N, Real E, Gaudin Y et al. Molecular basis for the interaction between rabies virus phosphoprotein P and the dynein light chain LC8: dissociation of dynein-binding properties and transcriptional functionality of P. J Gen Virol. 2001;82:2691–2696. doi: 10.1099/0022-1317-82-11-2691. [DOI] [PubMed] [Google Scholar]
- 36.Gupta AK, Blondel D, Choudhary S, Banerjee AK. The phosphoprotein of rabies virus is phosphorylated by a unique cellular protein kinase and specific isomers of protein kinase C. J Virol. 2000;74:91–98. doi: 10.1128/jvi.74.1.91-98.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchanan T. Rabies control in New Brunswick in 2001. The Rabies Reporter. 2002;12:4–8. [Google Scholar]