SUMMARY
In The Netherlands, a national programme for the surveillance of zoonotic bacteria in farm animals has been operative since 1997. We describe the results of the surveillance of Salmonella spp. in flocks of laying hens and broilers and of Campylobacter spp. in broiler flocks in the period 1999–2002. The prevalence of Salmonella spp. in laying-hen flocks has significantly decreased from 21·1% in 1999 to 13·4% in 2002. This decreasing trend might indicate that the control measures taken by the poultry industry were effective. S. Enteritidis was the predominant serovar in laying hens accounting for one third of the positive flocks. Although prevalence estimates for Salmonella spp. in broiler flocks did not yield a significant decreasing trend in 1999–2002, a decrease in Salmonella prevalence to 11% was measured in 2002. During the study period, S. Paratyphi B var. Java emerged in broilers to become the predominant serovar in 2002 accounting for one third of the positive flocks. The prevalence of Campylobacter spp. in broiler flocks did not increase nor decrease continuously between 1999 and 2002, which roughly corresponds with the monitoring results from the poultry industry. In this period, the estimated flock prevalence roughly averaged around 20%, with C. jejuni being the predominant species. The approach of monitoring presented in this paper can serve as a blueprint for monitoring schemes in farm animal populations to be developed in the context of the EC Zoonoses Directive.
INTRODUCTION
Salmonella and Campylobacter spp. remain the most common bacterial causes of human diarrhoeal illness in many countries [1]. In The Netherlands, in 1996–1999, a sentinel study on gastroenteritis in general practices was conducted in which Salmonella and Campylobacter spp. were associated with 4 and 10% of the cases respectively [2]. In addition, in 1999–2000, a study was conducted in the Dutch general population indicating the total number of cases of salmonellosis and campylobacteriosis to be approximately 50 000 and 100 000 respectively [3]. Whereas the number of laboratory-confirmed cases of campylobacteriosis in The Netherlands remained at a fairly constant level, the number of confirmed human Salmonella infections has declined consistently during the past decade [4].
For many decades, Salmonella enterica subspecies enterica serovar Typhimurium (S. Typhimurium) was the predominant Salmonella serotype in humans in The Netherlands. However, from 1988, Salmonella enterica serovar Enteritidis (S. Enteritidis) has emerged as a major serotype in man, with PT4 being the most prevalent phage type. In 1995, S. Enteritidis PT4 accounted for approximately 50% of Salmonella infections in humans [5], after which the contribution of this phage type has been more than halved to be replaced by other S. Enteritidis phage types as in many other European Union countries [6, 7]. S. Enteritidis infections have predominantly been associated with the consumption of raw eggs and egg-containing foods [8, 9]. In The Netherlands, the contribution of eggs to human cases of salmonellosis was estimated at approximately 35% between 1999 and 2002 [6], while more than 80% of the S. Enteritidis infections were associated with eggs (W. van Pelt, unpublished observations). Further, infections caused by other Salmonella serotypes have mainly been associated with consumption of (undercooked) foods of animal origin, and food animals, especially poultry, pigs and cattle, are considered major sources of infection [6].
The main Campylobacter species causing disease in humans are C. jejuni (accounting for the vast majority of the cases) and C. coli [10, 11]. In The Netherlands, C. jejuni and C. coli accounted for 89% and 8% respectively, of all laboratory-confirmed cases in 2002 (Y. Doorduyn, unpublished observations). In developed countries worldwide, Campylobacter infections in humans have mainly been associated with the consumption of undercooked poultry meat, handling of raw poultry, consumption of raw milk or untreated drinking water, direct contact with pets, foreign travel and, to a lesser extent, with consumption of meat other than poultry [12, 13]. In The Netherlands, 30·5, 32·5 and 31·3% of raw chicken products, including whole carcasses and parts of legs and breasts, sampled at retail in 2000, 2001 and 2002 respectively, were contaminated with campylobacters [14], whereas samples of raw pork and beef were rarely found to be contaminated [15].
In 1997, the Dutch Product Boards for Livestock, Meat and Eggs implemented monitoring and control programmes in the poultry meat and egg production chains to reduce Salmonella and Campylobacter contamination of poultry meat, and S. Enteritidis and S. Typhimurium contamination of laying hens [16, 17]. These programmes include amongst others microbiological examination of flocks at each stage of the production chain, application of strict hygiene measures throughout the production chain and a logistic slaughtering procedure for broiler flocks.
The EC Zoonoses Directive [18] obliges all Member States to report on the occurrence of zoonoses and zoonotic agents annually. From this perspective, in 1997, the Dutch Food and Consumer Product Safety Authority/Inspectorate for Health Protection and Veterinary Public Health (VWA/KvW) commissioned the National Institute for Public Health and the Environment (RIVM) to implement a surveillance programme in farm animals in The Netherlands with the main objective to monitor trends in the occurrence of zoonotic bacteria. In this report, the results of the surveillance of Salmonella spp. in flocks of laying hens and broilers and of Campylobacter spp. in broiler flocks in the period 1999–2002 are described. These results were also used to measure the effects of the control programmes in the poultry industry.
MATERIALS AND METHODS
Programme design
A two-stage sampling scheme was used to accurately estimate the annual prevalences of Salmonella and Campylobacter spp. at flock level. Each year, the primary sample sizes (number of laying-hen and broiler flocks to be sampled) were calculated for estimation of the predicted prevalences with an accuracy of 5% at a 90% confidence level. This confidence level was chosen to limit the number of sampling units from a logistic point of view. The calculations were made by using the epidemiological computer program WinEpiscope [19]. Appropriate numbers of laying-hen and broiler farms were randomly selected by the Animal Health Service (GD) from a national database stratified according to geographical region. The selected farmers were requested to participate in the voluntary programme. Sampling was conducted by employees of the VWA/KvW according to a sampling protocol. On each farm, one flock (i.e. all birds of similar age housed within one building) was randomly selected for sampling. From the selected flock, 60 samples were taken enabling detection of at least 5% shedding animals at a 95% confidence level. Fresh faecal samples were randomly collected from the floor or – in case of laying hens – the manure conveyer and, subsequently, the samples were aggregated into five pooled samples. The samples were transported to the RIVM in cooled transport boxes by a professional delivery service. Microbiological examination started within 48 h. Approximately 100–200 flocks of laying hens and broilers were sampled annually. However, due to an epidemic of foot-and-mouth disease (FMD), no samples were obtained from March 2001 until July 2001.
Detection of Salmonella spp.
For the detection of Salmonella spp. in faecal samples a modification of ISO 6579 [20] was applied, using a combination of Rappaport–Vassiliadis (RV) broth and modified semi-solid Rappaport–Vassiliadis (MSRV) agar for selective enrichment. In summary, from each pooled sample, 25 g was added to 225 ml of buffered peptone water [Nederlands Vaccin Instituut (NVI), Bilthoven, The Netherlands] and incubated for 18±2 h at 37±1°C. Of the pre-enrichment culture, 0·1 ml was inoculated in 10 ml RV broth (Oxoid, Haarlem, The Netherlands), which was incubated for 24±2 h at 42±1°C. The culture obtained in RV broth was plated on Brilliant Green Agar (BGA; Oxoid), followed by incubation for 24±2 h at 37±1°C. If no suspect colonies were obtained on BGA, the RV culture was plated out on BGA again after a second incubation period of 24±2 h at 42±1°C. Preparation of MSRV (Difco; Becton Dickinson, Alphen a/d Rijn, The Netherlands), containing 0·01 g l−1 novobiocine (Sigma-Aldrich Chemie, Wyndrecut, The Netherlands), was performed according to the manufacturer's instructions. Three drops of the pre-enrichment culture were inoculated on this medium, followed by incubation for 2×24±2 h at 42±1°C. Suspect white culture from the border of the growth zone was transferred onto BGA and handled as described for the RV procedure. Suspect colonies on BGA were biochemically confirmed using ureum agar with triple sugar iron agar (NVI) and lysine-decarboxylase broth (NVI). Serotyping of Salmonella isolates and phage-typing of S. Enteritidis and S. Typhimurium isolates were performed according to the standard operating procedures of the Dutch National Reference Laboratory for Salmonella (RIVM, Bilthoven). Quantitative antimicrobial resistance testing of Salmonella isolates was performed at the Dutch National Reference Laboratory for antimicrobial resistance testing (Central Institute for Animal Disease Control, Lelystad).
Detection of Campylobacter spp.
For the detection of thermophilic Campylobacter spp., each pooled faecal sample was directly plated on Campylobacter blood-free selective agar (CCDA; Oxoid) using sterile swabs, followed by incubation for 48±4 h at 42±1°C using the GENbox Microaer system (bioMérieux, Marcy l'Etoile, France). Subsequently, plates were examined for the presence of suspect colonies and if present, one colony per plate was transferred to CCDA, inoculating colonies from positive pooled samples from the same flock on the same plate. The microaerobic incubation procedure was repeated and characteristic Campylobacter colonies were examined by microscope for typical spiral-shaped cells and rapid motility. If the microscopic results were ambiguous, the Indx Campy agglutination test (Bipharma, Weesp, The Netherlands) was performed additionally, as prescribed by the manufacturer. Up to 2001, one Campylobacter isolate per positive pooled sample was stored in 2 ml peptone glycerol. At a later stage, these isolates were subjected to the mixed polymerase chain reaction (PCR) method described by Van de Giessen et al. [21] to discriminate C. coli from C. jejuni. In 2002, the species of the Campylobacter isolates were not determined.
Data analyses
Annual prevalence estimates, standard errors and confidence limits were calculated by using the methods provided by Thrusfield [22]. Trend analyses were performed using multivariable logistic regression (MLR), by analysing a continuous ‘trend-variable’ that represented subsequent time periods of 3 months. Applying MLR enabled adjustments for other factors, such as differences in age or flock size distribution between years and the non-sampling period in 2001, thereby excluding possible biases in the prevalence estimates. A trend was assumed to be present if the likelihood ratio test for the trend-variable yielded a significant P value at the 95% confidence level. The qualitative geographical analyses were performed by using the SAS/GIS procedure in the statistical software package SAS [23]. Statistics Netherlands [24] provided the geographical spread of farms in The Netherlands that were used for comparison with the geographical spread of sampled farms. The statistical tests for assessing differences between the geographical distribution of farms in the database and the actual distribution of farms in The Netherlands were based on χ2 distribution.
RESULTS
For both laying hens and broilers, the geographical distribution of the sampled flocks corresponded well with the actual distribution of farms in The Netherlands. No spatial clustering of positive flocks was observed. Salmonella- and Campylobacter-positive flocks were observed throughout the country. For illustration, the geographical distribution of the broiler flocks sampled in 2001 is illustrated in Figure 1.
Fig. 1.
Geographical distribution of the sampled broiler flocks compared to the distribution of broiler farms in The Netherlands in 2001.
In Table 1, the crude annual prevalence estimates for Salmonella and Campylobacter spp. as well as their 90% confidence intervals are presented numerically. A graphical representation of the crude annual prevalence estimates for Salmonella spp. in laying hens is given in Figure 2. A decrease was observed from 21% of positive flocks in 1999 to 13% in 2002. Trend analysis on the data adjusted for age, flock size and the non-sampling period due to the FMD outbreak in 2001, showed a significant decreasing trend (P=0·02) within this 4-year period. The prevalence of S. Enteritidis was a fairly constant fraction of approximately 45% of the Salmonella-positive flocks in all years, with the exception of 2001 (33%). Trend analysis on the prevalence estimates for S. Enteritidis yielded a P value of 0·08 for a decreasing trend, thus suggesting significance at the selected confidence level. Figure 3 shows the percentage of Salmonella- and S. Enteritidis-positive flocks according to time present at the farm indicating an increase of Salmonella-positive flocks in the last part of the production period. The most frequently isolated Salmonella serotypes are shown in Table 2. In laying hens, S. Enteritidis was the predominantly isolated serotype, PT4 being the predominant S. Enteritidis phage type.
Table 1.
Crude annual prevalence estimates (P) for Salmonella and Campylobacter spp. in Dutch poultry production flocks

CI, Confidence interval.
n gives the number of flocks examined that year.
Fig. 2.
Crude annual prevalence estimates (with standard errors) for Salmonella spp. (–■–) and S. Enteritidis (- -◆- -) in flocks of laying hens. * Inaccurate estimate caused by a non-sampling period from March to July 2001 due to an epidemic of foot-and-mouth disease.
Fig. 3.
Percentage of sampled laying-hen flocks and percentage (with standard errors) of Salmonella-positive (–●–) and S. Enteritidis-positive (- -◆- -) flocks according to time present at the farm.
Table 2.
Most frequently isolated Salmonella serotypes in Dutch poultry production flocks
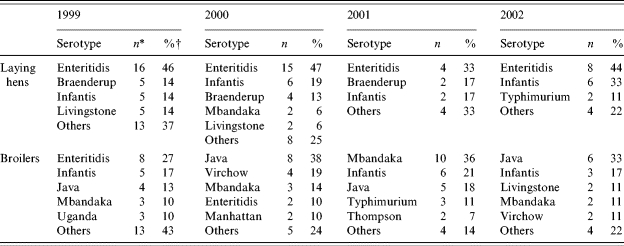
n gives the number of flocks positive for this serotype.
Percentage of the total number of Salmonella-positive flocks.
The crude annual prevalence estimates for Salmonella spp. in broiler flocks are illustrated in Figure 4. The estimates showed comparable values between 1999 and 2001, followed by a decrease in 2002. Trend analysis on data from 1999 until 2002, adjusted for age, flock size and the non-sampling period in 2001, did not show a statistically significant decreasing trend for Salmonella spp. (P=0·20). In 2001–2, the predominant Salmonella serotypes in broiler flocks were S. Mbandaka, S. Infantis and S. Paratyphi B var. Java (Table 2). The latter serotype increased from 13% of the Salmonella-positive flocks in 1999 to one third of the positive flocks in 2002. Multi-resistant S. Typhimurium DT104 (corresponding to the Dutch phage types 406 and 501) was isolated from two broiler flocks in 2001 and from one in 2002. Figure 5 shows the percentage of Salmonella- and Campylobacter-positive flocks according to age. Whereas the percentage of Salmonella-positive flocks declined after 3 weeks of age, the percentage of Campylobacter-positive flocks steadily increased towards the end of the broiler period.
Fig. 4.
Crude annual prevalence estimates (with standard errors) for Salmonella spp. (–■–) and S. Paratyphi B var. Java (–◆–) in broiler flocks. * Inaccurate estimate caused by a non-sampling period from March to July 2001 due to an epidemic of foot-and-mouth disease.
Fig. 5.
Percentage of sampled broiler flocks and percentage (with standard errors) of Salmonella-positive (–●–) and Campylobacter-positive (- -◆- -) flocks according to age.
The crude annual prevalence estimates for Campylobacter spp. in broiler flocks are illustrated in Figure 6. In 2000 and 2002, an increase in the prevalence estimate was observed compared to 1999 and 2001 respectively. Trend analysis of the prevalence estimates, adjusted for age, flock size, season and the non-sampling period due to FMD, gave no indication for a significant trend during the study period (P=0·18). A seasonal variation in Campylobacter occurrence in broiler flocks was observed with a peak in the summer months (Fig. 6). Molecular analysis of the Campylobacter isolates revealed that C. jejuni was isolated from 85 to 100% of the positive flocks in different years, whereas C. coli was found to be present in 13–20% of the positive flocks.
Fig. 6.
Crude annual prevalence estimates (with standard errors) and seasonal variation of Campylobacter spp. in broiler flocks. * Inaccurate estimate caused by a non-sampling period from March to July 2001 due to an epidemic of foot-and-mouth disease. - -□- -, Per year;  , per month.
, per month.
DISCUSSION
In this surveillance study, basic statistical principles were applied for prevalence estimation of zoonotic bacteria in poultry production flocks. However, several factors may have affected the prevalence estimates. First, the willingness of farmers to participate in this voluntary surveillance programme might have been related to the farm or flock status and thus may have interfered with the randomization of the sample. Furthermore, positive flocks with a low percentage of shedding animals may have falsely tested negative due to the limited number of samples taken per flock. Also, the crude prevalence estimates have not been adjusted for misclassification of flocks due to imperfect diagnostic testing. Since the isolates were confirmed and subjected to serotyping or species differentiation, false-positive test results are unlikely. However, false-negative testing may have occurred, especially in case of low numbers of bacteria present in the pooled samples. Therefore, the presented crude prevalences are likely to underestimate the true prevalence. Nevertheless, by applying the same sampling scheme and the same sampling and testing methods in consecutive years and by adjusting the data for those factors that may cause biases in the estimates, trends in the prevalence of the target bacteria in poultry production flocks could be analysed.
From 1999 onwards, a significantly decreasing trend in the prevalence of Salmonella spp. in flocks of laying hens was observed. As regards S. Enteritidis, the limited number of positive flocks might have caused the absence of statistical significance. The estimated Salmonella prevalence in 2002 was 13%, with S. Enteritidis accounting for one third of the positive flocks. The decreasing trend for Salmonella spp. (and a similar tendency for S. Enteritidis) in laying-hen flocks might indicate that the control measures taken by the poultry industry are effective. However, the initial aim of the control programme to reduce Salmonella contamination in laying hens to <5% of S. Enteritidis- or S. Typhimurium-positive flocks was not achieved until now. Furthermore, serological examination of laying-hen flocks at the end of production by the Animal Health Service (GD) indicated a decrease in S. Enteritidis-positive flocks from 14% in 1997 to 9% in 2002 [6]. The results of this serological monitoring roughly correspond with the results of our surveillance programme, indicating a decrease of S. Enteritidis infections in flocks of laying hens. Moreover, the increase in the percentage of Salmonella-positive flocks observed in the last part of the production period may well be due to the ageing of the hens, which thereby become more susceptible to infection.
In 1999, an extensive study on the prevalence of Salmonella spp. in table eggs in The Netherlands was conducted, yielding an estimate of at least 0·03% and at most 0·3% (14 pools of 10 eggs each out of 4620 pools were found positive). Eleven (77%) of the isolates were S. Enteritidis [25]. Since 2002, according to Dutch regulations, table eggs are required to be derived from laying-hen flocks free of Salmonella spp., and consequently the Dutch control programme in the egg production chain aims to reduce S. Enteritidis and S. Typhimurium infections in laying hens to zero level. In the United Kingdom in 2003, a survey of Salmonella contamination of table eggs revealed that 0·3% of the eggs were contaminated with Salmonella spp. This is a threefold reduction in the level of contamination since 1995/1996 and may well be attributable to the Salmonella control measures introduced by the UK egg industry, which included vaccination of laying hens [26].
In this study, PT4 was found to be the predominant S. Enteritidis phage type in laying hens and not surprisingly, in human S. Enteritidis infections during the study period as well. However, the contribution of PT4 to human S. Enteritidis infections declined from 80% in 1997 to 50% in 2002 [6]. In contrast, other S. Enteritidis phage types such as PT21 and PT1 emerged in humans between 1997 and 2002, whereas these phage types were rarely isolated from laying hens in this study. The emergence of phage types uncommon in Dutch laying hens may be related to the import of table eggs from other European countries. In 2003, an exceptional increase of human S. Enteritidis infections in The Netherlands was associated with the import of Spanish table eggs due to the avian influenza crisis in the Dutch poultry industry [27]. Also, in a recent 2-year Health Protection Agency survey of eggs derived from food premises and suppliers implicated in outbreaks of Salmonella spp., 5·6% of Spanish eggs were found to be contaminated with Salmonella spp. compared to 1·1% of eggs derived from non-vaccinated laying-hen flocks in the United Kingdom [28]. This development highlights the urgent need for the control of Salmonella spp. in laying hens and eggs at the European level.
Trend-analysis on the Salmonella prevalences measured in broiler flocks in 1999–2002 did not yield a decreasing trend (P=0·20). However, the decrease in Salmonella prevalence observed in 2002 as well as the decreasing contamination percentages measured in the poultry meat production chain by the poultry industry [6] and in poultry products at retail by the KvW [14] suggest that the intervention measures implemented by the poultry industry are effective. A comparison of the results from the different Dutch monitoring programmes in the poultry meat production chain combined with the results of the public health laboratory surveillance is shown in Figure 7. The Salmonella prevalences in broiler flocks measured by the poultry industry are structurally lower than those measured in our surveillance programme, which may be due to differences in sample size, sampling methods and analytical performance.
Fig. 7.
Comparison of Salmonella prevalences as measured in (i) the RIVM monitoring programme in broiler flocks (–■–), (ii) the poultry industry's (PVE) monitoring programme in broiler flocks (–◆–) and breast skins (–●–) [6], (iii) the monitoring programme of the Inspectorate for Health Protection and Veterinary Public Health (KvW) in chicken end-products at retail (–▲–) [14] and (iv) the public health laboratory surveillance in human stool specimens (
 ) [6].
) [6].
During the study period, the emergence of S. Paratyphi B var. Java (S. Java) in broilers was observed, corresponding with an explosive increase of this particular serotype in poultry products at retail in The Netherlands [29]. Simultaneously, a similar emergence of S. Java in poultry was observed in Germany [30]. Characterization of S. Java isolates from Dutch and German poultry revealed the clonal spread of a strain multi-resistant to antimicrobial drugs [29]. Farm intervention studies indicated a high level of persistence of S. Java in contaminated broiler houses (N. M. Bolder, unpublished observations). Moreover, the S. Java clone in broilers was found to rapidly develop resistance to quinolones, presumably due to veterinary treatment [31]. Since in The Netherlands fluoroquinolones are the antibiotics of first choice in cases of human salmonellosis, quinolone resistance might hamper effective medical treatment. Fortunately, up to now, S. Java infection in humans in The Netherlands has been rare, despite its abundance on poultry meat.
Prevalence estimates for Campylobacter spp. in broiler flocks averaged around 20% in the period 1999–2002. The absence of a decreasing (or increasing) trend corresponds with the results of the Campylobacter monitoring programme run by the poultry industry [6] and with the results of the KvW monitoring of poultry products at retail [14]. Campylobacter contamination of poultry products at retail in The Netherlands was 23·5% in 1998 and 31·3% in 2002. Although these contamination levels are relatively low compared to those measured in some other European countries like Denmark (41·7%) and France (88·7%) in 2002 [1], these results indicate that the Dutch control programme implemented in the poultry meat production chain was unsuccessful in reducing the Campylobacter contamination levels. Therefore, the application of alternative intervention measures including channeling of infected flocks in combination with germicidal treatment of poultry carcasses, such as decontamination, freezing or irradiation, should be seriously considered. To this end, in The Netherlands, a national Campylobacter risk management and assessment project was launched in 2001 and completed in 2004 [32].
In contrast to Salmonella spp. for which a decline in the percentage of positive broiler flocks was observed after 3 weeks of age, a steady increase in the percentage of Campylobacter-positive flocks was observed during the broiler period, which may be attributable to the transmission of Campylobacter from environmental sources at different ages followed by the characteristic rapid spread of the bacterium within the flock [33].
The seasonal pattern for Campylobacter occurrence in broiler flocks showed a peak in the period May–July, which corresponds with a peak in the Campylobacter contamination of poultry products in the period June–August. Although a similar seasonal variation is observed in human campylobacteriosis in The Netherlands, the relation between the summer peaks in Campylobacter occurrence in humans and poultry is uncertain due to a large discrepancy between the levels of fluoroquinolone resistance in human and poultry isolates in the summer period [34]. The phenomenon of seasonal variation of Campylobacter in broilers is also observed in other countries and may be related to climatic conditions influencing the environmental infection pressure of Campylobacter spp. [35].
The majority of the Campylobacter isolates from broilers were C. jejuni, which corresponds with findings from other studies [21, 36]. Moreover, C. jejuni is the predominant species causing human campylobacteriosis in The Netherlands [2].
The surveillance approach presented in this report demonstrated its worth in monitoring and evaluating trends in the occurrence of multiple zoonotic agents in farm animal populations. This approach can serve as a blueprint for monitoring schemes in farm animals to be developed in the context of the EC Zoonoses Directive.
ACKNOWLEDGEMENTS
This study was conducted by order of the Dutch Food and Consumer Product Safety Authority (VWA). We thank our colleagues at the Inspectorate for Health Protection and Veterinary Public Health (KvW/VWA) for taking the samples and the Animal Health Service (GD) for selecting the farms and contacting the selected farmers. Thanks are also due to Dr A. Mensink for a critical review of the paper.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Anon Brussels: European Commission; 2005. . Trends and sources of zoonotic agents in animals, feedstuffs, food and man in the European Union and Norway in 2003. . SANCO/339/2005. [Google Scholar]
- 2.De Wit MA et al. Gastroenteritis in sentinel general practices, the Netherlands. Emerging Infectious Diseases. 2001;7:82–91. doi: 10.3201/eid0701.010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Wit MA et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. American Journal of Epidemiology. 2001;154:666–674. doi: 10.1093/aje/154.7.666. [DOI] [PubMed] [Google Scholar]
- 4.Pelt W van et al. Laboratory surveillance of bacterial gastroenteric pathogens in The Netherlands, 1991–2001. Epidemiology and Infection. 2003;130:431–441. [PMC free article] [PubMed] [Google Scholar]
- 5.Giessen AW van de, Leeuwen WJ van, Pelt W van, Saeed AM, Gast RK, Potter ME, Wall PG. Salmonella enterica serovar Enteritidis in Humans and Animals: epidemiology, pathogenesis and control. Ames, IA: Iowa State University Press; 1999. Salmonella enterica serovar Enteritidis in the Netherlands: epidemiology, prevention and control; pp. 71–80. : pp. [Google Scholar]
- 6.Anon The Hague: Dutch Food and Consumer Product Safety Authority; 2004. . Report of trends and sources of zoonotic agents. The Netherlands, 2003. [Google Scholar]
- 7.Fisher IST. International trends in Salmonella serotypes 1998–2003 – a surveillance report from the Enter-net international surveillance network. Eurosurveillance. 2004;9:9–10. doi: 10.2807/esm.09.11.00487-en. [DOI] [PubMed] [Google Scholar]
- 8.Van Duynhoven YTHP et al. A one-year intensified study of outbreaks of gastroenteritis in the Netherlands. Epidemiology and Infection. 2005;133:9–21. doi: 10.1017/s0950268804002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Health Protection Agency. National increase in Salmonella Enteritidis outbreaks. Communicable Disease Report Weekly. 2003;13(35):4. [Google Scholar]
- 10.Skirrow MB. Campylobacter. Lancet. 1990;336:921–923. doi: 10.1016/0140-6736(90)92282-m. [DOI] [PubMed] [Google Scholar]
- 11.Tauxe RV, Nachamkin I, Blaser MJ, Tompkins LS. Campylobacter jejuni Current Status and Future Trends. Washington, DC: American Society for Microbiology; 1992. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations; pp. 9–19. : pp. [Google Scholar]
- 12.Altekruse SF et al. Campylobacter jejuni – an emerging foodborne pathogen. Emerging Infectious Diseases. 1999;5:28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allos BM. Campylobacter jejuni infections: update on emerging issues and trends. Clinical Infectious Diseases. 2001;32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 14.Van der Zee H, Wit B Zutphen: Inspectorate for Health Protection and Veterinary Public Health; 2003. . Monitoring pathogens in poultry and poultry products, 2002 [in Dutch]. [Google Scholar]
- 15.Van der Zee H, Wit B, de Boer E. Survey of pathogenic micro-organisms in raw meats [in Dutch] De Ware(n)-Chemicus. 2000;30:185–188. [Google Scholar]
- 16.Anon. Action plan Salmonella and Campylobacter in the poultry meat sector 2000+ [in Dutch] Rijswijk: Product Boards for Livestock, Meat and Eggs; 2000. [Google Scholar]
- 17.Anon. Action plan Salmonella in the egg sector 2001+ [in Dutch] Rijswijk: Product Boards for Livestock, Meat and Eggs; 2001. [Google Scholar]
- 18.Anon. Directive 2003/99/EC of the European parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. Official Journal of the European Union 2003:31–40. , 12 Dec. , L325: pp. [Google Scholar]
- 19.Frankena K et al. EPISCOPE: computer programs in veterinary epidemiology. Veterinary Record. 1990;126:573–576. [PubMed] [Google Scholar]
- 20.Anon. Microbiology – general guidance on methods for detection of Salmonella. 3rd edn. Geneva: International Standard Organization; 1993. ; ISO 6579. [Google Scholar]
- 21.Van de Giessen AW et al. Reduction of Campylobacter infections in broiler flocks by application of hygiene measures. Epidemiology and Infection. 1998;121:57–66. doi: 10.1017/s0950268898008899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thrusfield M. Veterinary Epidemiology. 2nd edn. Oxford: Blackwell Science; 1995. p. 483. : p. [Google Scholar]
- 23.SAS Institute Inc Cary, NC: SAS Institute Inc.; 2001. . SAS version 8.02. [Google Scholar]
- 24.Statistics Netherlands . Voorburg, 2002. StatLine (http:/statline.cbs.nl/StatWeb). 2003.
- 25.De Boer E, Wit B. Salmonella in eieren. Tijdschrift Voor Diergeneeskunde. 2000;124:126–127. [PubMed] [Google Scholar]
- 26.Elson R, Little C. First United Kingdom-wide study of raw shell eggs and their use in catering premises published. www.eurosurveillance.org/ew/2004/040318.asp Eurosurveillance Weekly releases. 2004;8 (12): 040318 ( [Google Scholar]
- 27.Pelt W van et al. An explosion of Salmonella infections in 2003 in the Netherlands: hot summer or side effect of the avian influenza outbreak? Eurosurveillance. 2004;9:3–4. doi: 10.2807/esm.09.07.00473-en. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien S et al. Increase in Salmonella Enteritidis outbreaks in England and Wales. Eurosurveillance Weekly. 2003;7:28. August 2005. [Google Scholar]
- 29.Pelt W van et al. Explosive increase of Salmonella Java in poultry in the Netherlands: consequences for public health. Eurosurveillance. 2003;8:31–35. doi: 10.2807/esm.08.02.00398-en. [DOI] [PubMed] [Google Scholar]
- 30.Dorn C et al. Increasing number of Salmonella paratyphi B isolates from slaughtered poultry sent in to the National Salmonella Reference Laboratory [in German] Berliner und Munchener Tierarztliche Wochenschrift. 2001;114:179–183. [PubMed] [Google Scholar]
- 31.Mevius DJ, Pelt W van. The Hague: Dutch Food and Consumer Product Safety Authority; 2003. [Google Scholar]
- 32.Havelaar AH. Bilthoven, The Netherlands: National Institute for Public Health and the Environment; 2001. . Report ID 250911001/2001. [Google Scholar]
- 33.Newell DG, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Applied and Environmental Microbiology. 2003;69:4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelt W van et al. Resistance to fluoroquinolones marking the origin of human Campylobacter infections in the Netherlands. Clinical Microbiology and Infection. 2001;7:308. [Google Scholar]
- 35.Wallace JS et al. Seasonality of thermophilic Campylobacter populations in chickens. Journal of Applied Microbiology. 1997;82:219–224. [PubMed] [Google Scholar]
- 36.Refrégier-Petton J et al. Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Preventive Veterinary Medicine. 2001;50:89–100. doi: 10.1016/s0167-5877(01)00220-3. [DOI] [PubMed] [Google Scholar]









