SUMMARY
A case-control study was performed in South Australia to determine if L. longbeachae infection was associated with recent handling of commercial potting mix and to examine possible modes of transmission. Twenty-five laboratory-confirmed cases and 75 matched controls were enrolled between April 1997 and March 1999. Information on underlying illness, smoking, gardening exposures and behaviours was obtained by telephone interviews. Recent use of potting mix was associated with illness (OR 4·74, 95% CI 1·65–13·55, P=0·004) in bivariate analysis only. Better predictors of illness in multivariate analysis included poor hand-washing practices after gardening, long-term smoking and being near dripping hanging flower pots. Awareness of a possible health risk with potting mix protected against illness. Results are consistent with inhalation and ingestion as possible modes of transmission. Exposure to aerosolized organisms and poor gardening hygiene may be important predisposing factors to L. longbeachae infection.
INTRODUCTION
In Australia, Legionella longbeachae infections have been reported since 1987 [1] and are notified as often as L. pneumophila infections [2]. L. longbeachae infections occur more frequently during springtime [3]. However, there is not a uniform pattern of disease incidence across the country and South and Western Australia consistently report greater proportions (over 80%) of legionellosis due to L. longbeachae infection [2].
Although L. longbeachae has a clinical picture indistinguishable from other Legionella species, there is less epidemiological evidence of risk factors and possible modes of transmission for L. longbeachae than for L. pneumophila. Results from an investigation into 22 cases of L. longbeachae infection in South Australia (SA) during 1988–1989, found cases were regular gardeners and a common feature of their gardens was the presence of ferneries with hanging baskets [4]. L. longbeachae was subsequently isolated from cases' potting mix (which consisted mainly of composted pine bark), providing a plausible natural habitat for this bacterium [5]. Further links between L. longbeachae infection and potting mix have been made in Australia, Japan and the United States, through case-series and laboratory evidence [6–9]. Transmission of L. longbeachae has been proposed through mechanisms common to other Legionella species, specifically inhalation of contaminated aerosols, and ingestion (via contaminated hands) then microaspiration of organisms [10, 11].
The primary aim of the study was to determine if L. longbeachae infection was associated with handling of commercial potting mix. We also examined possible mechanisms of transmission including inhalation of contaminated dust and aerosols and, ingestion of organisms via contaminated hands.
METHODS
Study design and population
We conducted a matched case control study in SA between April 1997 and March 1999 using 25 cases and 75 population-based controls. A case was defined as a person notified to the SA Communicable Disease Control Branch during the same period, with a clinically compatible illness consisting of fever, cough or pneumonia in addition to laboratory confirmation of L. longbeachae infection by culture or serology (a ⩾fourfold rise in titre, to at least 128). Controls were selected from a database containing a representative sample of South Australian households and interviewed using Computer Assisted Telephone Interviews (CATI) [12]. Controls were matched for age (±5 years), sex and postal area of residence or geographically adjacent postcode. Potential controls were excluded if they had developed a fever, cough, chest pain, or diarrhoea of at least 1 day's duration within the previous month.
Study instrument and measures
The study instrument was a structured questionnaire that was previously piloted and administered via telephone by two trained interviewers. Baseline information was obtained on pre-existing medical conditions (cardiovascular disease, respiratory conditions, diabetes, immunosuppression or other medical conditions), history of travel in the previous 4 weeks and current and previous smoking behaviour.
Information was obtained from cases and controls on frequency of gardening, proximity to gardening areas and enclosed areas (including hot-houses and ferneries), recent use of garden soil (including compost, manure and potting mix), exposure to landscaping and proximity to general garden watering and hanging pots dripping with water in the previous 4 weeks. Data on respondent health behaviours associated with gardening included smoking while gardening, hand-washing practices, and wearing gloves or face mask. Additional information was obtained on the individual's awareness of the possible health risk associated with use of potting mix. Information on exposures was obtained for the 4 weeks prior to hospitalization for cases and 4 weeks prior to interview for controls.
Statistical analysis
The data were entered into Epi-Info version 6 (CDC, Atlanta, GA, USA) and analysed using stata version 8 (StataCorp., College Station, TX, USA), and StatXact/LogXact program (Cytel Software Corp., Cambridge, MA, USA) where data were sparse. A matched analysis was performed using conditional logistic regression. Bivariate analysis was undertaken initially to examine associations between all exposure variables and L. longbeachae. Next, a multivariate logistic model was developed and included all variables in the bivariate analyses with P values <0·25 [13]. A backward stepwise elimination method using all variables was attempted, however, the sparseness of data would not support this method. As such, the best predictors were selected from each of five exposure groups: pre-existing medical conditions, smoking history, gardening frequency and activities (including use of soils, compost, manure, potting mix and landscaping), risk awareness and gardening behaviours. The main exposure (recent potting mix use) along with the best predictor variables from the other four exposure groups (pre-existing medical conditions, smoking history, risk awareness and gardening behaviours) were modelled against illness, the outcome variable. To determine the best predictors of illness overall, each of the five exposure predictors were modelled against illness in a step-wise backward elimination of non-significant variables, based on log-likelihood ratio tests and a P value of 0·05. Correlation between variables was assessed using 2×2 tables and interaction (between variables used in the multivariate model) was tested using the likelihood ratio test. Crude and adjusted odds are reported together with 95% confidence intervals.
RESULTS
There was no difference in age and gender between cases and controls (Table 1). However cases had significantly more pre-existing cardiac, respiratory and other medical conditions. Cases were also more likely to have smoked, including long-term smoking.
Table 1.
Baseline characteristics of cases and controls
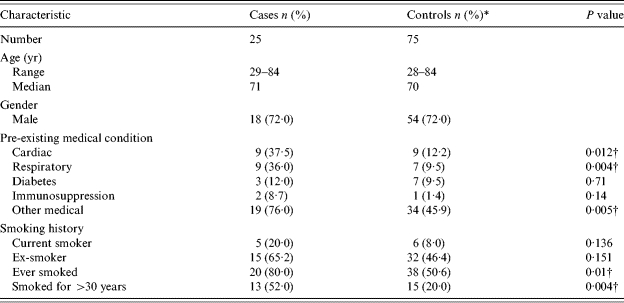
Percentage of people who answered.
Statistically significant at the P<0·05 level.
Compared to controls, cases of L. longbeachae infection were more likely to have underlying cardiac, respiratory and other medical conditions (Table 2). The most commonly reported other medical condition was hypertension (30·8%). Cases were also more likely to have ever smoked than controls. Smokers (either current or previous) were significantly more likely to have experienced L. longbeachae illness.
Table 2.
Matched analysis of underlying medical conditions and smoking exposure – crude odds and 95% confidence intervals for likelihood of L. longbeachae infection
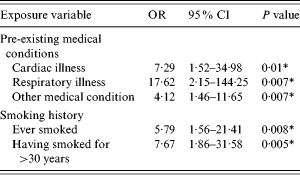
OR, Odds ratio; CI, confidence interval.
Statistically significant at the P<0·05 level.
Use of potting mix, in pots or anywhere else in the garden, in the 4 weeks prior to hospitalization was significantly associated with illness [odds ratio (OR) 4·74, 95% confidence interval (CI) 1·65–13·55, P=0·004] (Table 3). Watering down potting mix before use (as is often advised as a dust suppression measure), was not found to offer protection against illness in this study (OR 0·14, 95% CI 0·0–1·04, P=0·08). Possible exposure to water-borne aerosolized L. longbeachae by being near hanging pots that were dripping increased the risk of illness (OR 2·79, 95% CI 1·05–7·47, P=0·04). However, other variables which could represent close proximity to garden soils such as making your own compost, use of compost and manure and exposure to rich soils in an enclosed environment, were not associated with illness in this study. Other gardening exposures such as gardening frequency and exposure to watering in the garden (as opposed to hanging pots) were also not significantly associated with illness.
Table 3.
Matched analysis of gardening exposures, risk awareness and gardening behaviours – crude odds and 95% confidence intervals for likelihood of L. longbeachae infection
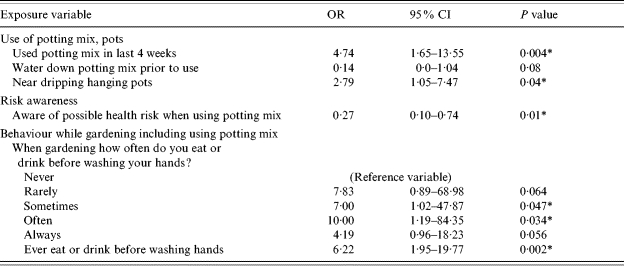
OR, Odds ratio; CI, confidence interval.
Statistically significant at the P<0·05 level.
Being aware of the possible health risk associated with use of potting mix was a significant protective factor against illness (Table 3). Of the gardening behaviours that were examined, eating or drinking after gardening without washing one's hands was associated with an increased likelihood of illness.
After adjusting for the effects of other exposure variables (pre-existing medical conditions, smoking history, risk awareness and gardening behaviours) in the model, use of potting mix was no longer significantly associated with illness. However, when other gardening activities were substituted for potting mix in the model, being near dripping, hanging pots remained significantly associated with illness.
The exposures that remain significant predictors of illness overall are eating or drinking after gardening before washing one's hands, smoking for more than 30 years and being near dripping hanging pots (Table 4). Being aware of a possible health risk when using potting mix remains a significant protector against illness. The final model indicated that these variables explain 60% of the variation in the data (pseudo R 2=0·6082). There was no correlation or significant interaction identified between variables.
Table 4.
Multivariate analysis – adjusted odds and 95% confidence interval for likelihood of L. longbeachae infection
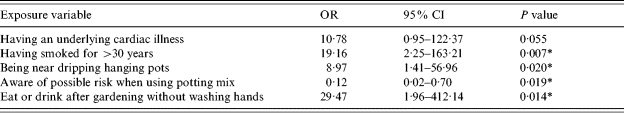
OR, Odds ratio; CI, confidence interval.
Statistically significant at the P<0·05 level.
DISCUSSION
The results from this study do not unequivocally support the hypothesis that L. longbeachae infection is associated with using potting mix in the 4 weeks before hospitalization. Instead, this study suggests there are other factors within the gardening environment, as well as intrinsic and behavioural host factors, that are better predictors of L. longbeachae infection than recent use of potting mix.
However, there was an indirect association between some of the significant predictors in the gardening environment and potting mix. Being near dripping, hanging pots is an important finding as it provides a potential source of the organism (pots often contain potting mix) and a potential mode of transmission through aerosolization. In addition, knowing of a possible health risk from using potting mix protected against illness. While it is not known how such knowledge may affect exposure to potting mix, it is possible that persons with this knowledge may handle potting mix differently to others. The statistical significance of these variables indicates that potting mix may still play a role in the epidemiology of L. longbeachae even though it did not remain significantly associated with illness in this study.
The host-related factors that were significant predictors of illness in this study are long-term smoking and poor gardening hygiene. These are newly described risk factors for L. longbeachae infection. Not only do they provide for a possible (oral) route of transmission, but smoking may also serve as a potential marker for underlying respiratory and cardiac disease. Cases have previously been described as being less likely to be current smokers and to have similar rates of chronic medical conditions as the general population [4]. However, the finding in the current study is consistent with such people having a higher risk of other Legionella infections such as L. pneumophila [14]. Poor gardening hygiene suggests that it may be how a person uses gardening soils, including potting mix, that is important in the development of L. longbeachae infection rather than the use of these soils as such.
The results provide important insights into the possible mechanisms for transmission of L. longbeachae. An association between illness and proximity to dripping, hanging pots supports inhalation of contaminated aerosols produced during watering as a possible mode of transmission. Another possible mode of transmission is ingestion of organisms via contaminated hands. This is supported by the association between illness and eating or drinking after gardening before washing hands. These results do not help to determine whether one of these methods of transmission is more important than the other and it is possible that both methods may be important for L. longbeachae infection.
Potential limitations in the study result from the sample size and the exposure window used. Retrospective calculations indicate that there was sufficient power to detect an association between potting mix use and L. longbeachae infection at the 0·05 level of significance; however the analysis of other gardening exposures and gardening behaviours was hindered due to the small sample size. A 4-week exposure window, rather than a time period closer to the 10-day incubation period, was used as there was less certainty about the incubation period when this study was designed 7 years ago. This may result in misclassification within the exposure variable as cases or controls reporting exposure between 10 days and 4 weeks before hospitalization or interview may have been misclassified as exposed when they may not have been. If cases and controls are equally as likely to report the timing of their exposure (within 10 days or between 10 days to 4 weeks) the results should be biased towards the null. Use of the 4-week period also introduces potential for poor recall due to cases trying to remember exposures up to 7 weeks after occurrence due to delays in interviewing cases after hospitalization (median 22 days, range 3–57 days). This is also likely to have resulted in bias towards the null.
There is also potential for selection bias as controls were initially respondents from a health survey who agreed to participate in further studies. This population is known to be overrepresented with people who are older, retired, better educated, and more likely to be health conscious [15, 16].
Another methodological issue is the potential for overmatching in this study. Although age may affect the potential for exposure as well as the potential for disease, it is unlikely that sex and postcode satisfy the criteria for confounders. Therefore, statistical inefficiency may have been introduced into the study by matching on variables that are only related to exposure [17].
Some results in this study are different to results from an earlier case-control study conducted in SA in 1988–1989. In the earlier study, cases were found to be frequent gardeners (at least 4 days a week) [4]. In contrast, in this later study, no association was found between frequency of gardening and illness. In fact, the exposure that was closest to statistical significance was gardening infrequently, or less than once a month. Having hanging pots was a common feature of cases' gardens in the earlier case-control study and remained a feature here, although it was not significantly different from controls. The presence of ferneries with hanging baskets and overhead watering systems, noted in cases' gardens in the earlier case-control study [4], is consistent with exposure to dripping, hanging pots being a significant predictor of illness in this study.
In conclusion, this study has provided some clarification of the risk factors for L. longbeachae infection in Australia. Firstly, recent use of potting mix as such was not found to be an independent risk factor for illness in this study. Rather, other factors in the garden were found to have greater importance, such as possible exposure to aerosolized L. longbeachae organisms from dripping hanging pots and poor hygiene in the garden. This study also has determined that long-term smokers have an increased risk of L. longbeachae infection and that awareness of a possible health risk from using potting mix helps to protect against illness.
Further information on risk factors for L. longbeachae infection that explores the interface between potting mix, other gardening exposures and behaviours while gardening need to be addressed in a larger study. This is essential to inform any public health message regarding the risk of L. longbeachae infection and to consolidate evidence for health warning labels that appear on bags of potting mix in Australia.
RECOMMENDATIONS
Long-term smokers and possibly people with pre-existing medical conditions such as respiratory and cardiac illness should be warned about their increased risk of L. longbeachae infection. Long-term smokers in particular should be advised to follow good hygiene when gardening and to wash hands before eating, drinking or smoking.
Raising people's awareness of a possible health risk when using potting mix should continue in order to protect against L. longbeachae infection.
This study should be used to calculate sample sizes for a further study to determine associations between specific gardening exposures, behaviours and L. longbeachae infection and to clarify the importance of various modes of transmission.
A further study should use an exposure window closer to the accepted incubation period of 10 days and consider an unmatched study design.
ACKNOWLEDGEMENTS
The authors acknowledge previous members of the Disease Surveillance and Investigation Unit of the Communicable Disease Control Branch, Department of Health South Australia for their role in conducting the study and the Epidemiology Branch for providing assistance with the interpretation of the results. The Master of Applied Epidemiology Program is funded by the Australian Government Department of Health and Ageing.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Lim I et al. Legionella longbeachae pneumonia: report of two cases. Medical Journal of Australia. 1989;150:599–601. doi: 10.5694/j.1326-5377.1989.tb136700.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller M et al. Australia's notifiable disease status, 2003 Annual report of the National Notifiable Diseases Surveillance System. Communicable Diseases Intelligence. 2005;29:1–76. [PubMed] [Google Scholar]
- 3.Li J, O'Brien E, Guest C. A review of national legionellosis surveillance in Australia, 1991–2000. Communicable Diseases Intelligence. 2002;26:461–468. [PubMed] [Google Scholar]
- 4.Cameron S et al. Epidemiological characteristics of Legionella infection in South Australia: implications for disease control. Australia and New Zealand Journal of Medicine. 1991;21:65–70. doi: 10.1111/j.1445-5994.1991.tb03007.x. [DOI] [PubMed] [Google Scholar]
- 5.Steele TW, Lanser J, Sangster N. Isolation of Legionella longbeachae serogroup 1 from potting mixes. Applied and Environmental Microbiology. 1990;56:49–53. doi: 10.1128/aem.56.1.49-53.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steele TW, Moore CV, Sangster N. Distribution of Legionella longbeachae serogroup 1 and other legionellae in potting soils in Australia. Applied and Environmental Microbiology. 1990;56:2984–2988. doi: 10.1128/aem.56.10.2984-2988.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabbay E et al. Legionella longbeachae in Western Australia: a 12-month retrospective review. Medical Journal of Australia. 1996;164:704. doi: 10.5694/j.1326-5377.1996.tb122261.x. [DOI] [PubMed] [Google Scholar]
- 8.Koide M, Arakaki N, Saito A. Distribution of Legionella longbeachae and other legionellae in Japanese potting soils. Journal of Infection and Chemotherapy. 2001;7:224–227. doi: 10.1007/s101560170017. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Legionnaires' Disease associated with potting soil – California, Oregon, and Washington, May–June 2000. Morbidity and Mortality Weekly Report. 2000;49:777–778. [PubMed] [Google Scholar]
- 10.Steele TW. The ecology of Legionella longbeachae in Australia. Medical Journal of Australia. 1996;164:703–704. doi: 10.5694/j.1326-5377.1996.tb122259.x. [DOI] [PubMed] [Google Scholar]
- 11.Blatt SP et al. Nosocomial legionnaires' disease: aspiration as a primary mode of disease acquisition. American Journal of Medicine. 1993;95:16–22. doi: 10.1016/0002-9343(93)90227-g. [DOI] [PubMed] [Google Scholar]
- 12.Population Research and Outcome Studies 2002. . The Social and Environmental Risk Context Information System (SERCIS) methodology, South Australian Department of Health. Brief report number 2002-11,
- 13.Hosmer DW, Lemeshow S. Applied Logistic Regression. USA: John Wiley & Sons; 1989. Chapter 4. Model-building strategies and methods for logistic regression; pp. 82–134. , pp. [Google Scholar]
- 14.Edelstein PH, Cianciotto NP, Mandell G, Bennett J, Dolin R. Principles and Practice of Infectious Diseases. Part III: Infectious Diseases and their Etiologic Agents. 6th edn. Philadelphia: Churchill Livingstone; 2005. Legionella; pp. 2711–2724. , pp. [Google Scholar]
- 15.Taylor AW, Wilson DH, Wakefield M. Differences in health estimates using telephone and door-to-door survey methods – a hypothetical exercise. Australian and New Zealand Journal of Public Health. 1998;22:223–226. doi: 10.1111/j.1467-842x.1998.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 16.Webb P, Bain C, Pirozzo S. Essential Epidemiology. New York: Cambridge University Press; 2005. All that glitters is not gold: the problem of error; pp. 148–182. , pp. [Google Scholar]
- 17.Schlesselman JJ. Case-Control Studies Design, Conduct, Analysis. New York: Oxford University Press; 1982. Chapter 4. Matching; pp. 105–123. , pp. [Google Scholar]


