SUMMARY
The Randomized Badger Culling Trial (RBCT) began in 1998 to determine the impact of badger culling in controlling bovine tuberculosis in cattle. A total of 1166 badgers (14% of total) proactively culled during the RBCT were found to be tuberculous, offering a unique opportunity to study the pathology caused by Mycobacterium bovis in a large sample of badgers. Of these, 39% of adults (~6% of all adults culled) had visible lesions (detectable at necropsy) of bovine tuberculosis; cubs had a lower prevalence of infection (9%) but a higher percentage of tuberculous cubs (55·5%) had visible lesions. Only ~1% of adult badgers had extensive, severe pathology. Tuberculous badgers with recorded bite wounds (~5%) had a higher prevalence of visible lesions and a different distribution of lesions, suggesting transmission via bite wounds. However, the predominance of lesions in the respiratory tract indicates that most transmission occurs by the respiratory route.
INTRODUCTION
Infection with Mycobacterium bovis, the causative agent of bovine tuberculosis (TB), continues to persist in the UK cattle population despite a national programme of regular herd testing to identify and remove infected animals having been in place for more than 50 years. The presence of M. bovis infection in badgers (Meles meles) in England was first found in 1971 and first published in 1974 [1] and infection was subsequently shown to be present in all regions where TB persisted in the cattle population [2]. Several studies involving necropsy of badgers have shown that a proportion of infected animals develop grossly visible pathological lesions, in some cases manifesting as disseminated disease [3–6]. Hence, the badger was implicated as a source of infection for cattle and consequently a number of badger-culling strategies were implemented from the mid-1970s to mid-1990s in an attempt to control the disease in cattle. Despite these interventions, the overall incidence of cattle herds affected by TB in England and Wales continued to rise during this period [7]. Following a review of the role of badgers in bovine TB in 1997 [7], the Randomized Badger Culling Trial (RBCT) was started in 1998 to provide a scientific evaluation of the effect of two badger-culling strategies on the incidence of TB in cattle. For full background and primary results from the RBCT see references [8–10].
Information on the distribution and extent of pathology in M. bovis-infected animals can provide insight into likely routes of bacterial excretion and transmission of infection. Studies in cattle involving experimental infection of animals by different routes have indicated that infection with M. bovis can occur by inhalation or ingestion, although the former route is more efficient [11, 12]. Consequently, infection usually establishes initially within the respiratory tract. Based on observations of the pathology in M. bovis-infected badgers, it has been suggested that infection in this species can also occur through bite wounds [6, 13]. Such infection via breaches in the skin might be expected to result in a distinctive pattern of pathology. The extent to which infection spreads from the initial site determines the severity and distribution of pathology, which can vary from a few small localized lesions to extensive pathology, sometimes involving a number of organ systems. It has been suggested that badgers with severe pathology and high bacterial loads have a particularly important role in transmitting infection to cattle [5].
Previous studies of the pathology caused by infection with M. bovis in badgers have reported widely varying values for the proportions of infected animals exhibiting visible gross pathology and those with severe disseminated pathology ([3, 4, reviewed in [5]). These studies, for the most part, relied on badgers removed from farms following cattle herd TB incidents and/or badger carcases found dead on the roads or on farmland. None of these samples of animals is necessarily representative of the local populations of badgers and therefore these samples might provide biased information on the incidence and severity of disease. One of the culling strategies tested in the RBCT, referred to as proactive culling, involved extensive removal of badgers on an annual basis from areas with a high incidence of TB in cattle. The use of cage trapping and dispatch by gunshot allowed the collection of badger carcases for necropsy, thus providing the opportunity to obtain quantitative information on the pathology associated with M. bovis infection in a large sample of badgers from areas with a high risk of bovine TB.
This paper presents quantitative information on the prevalence, distribution and severity of pathological lesions in this large sample of animals (1166 tuberculous adults and cubs) and discusses the factors that may influence lesion distribution and hence could potentially affect the transmission dynamics of M. bovis in the badger population.
METHODS
Culling of badgers
The first RBCT proactive cull was conducted in 1998 and the last in 2005. Thirty areas of land each of about 100 km2, in regions with a high incidence of TB in cattle, were chosen and allocated into 10 triplets of three trial areas each. Three different treatments were implemented in the RBCT: survey-only (a control group), reactive (localized) culling and proactive culling, and each was replicated in each triplet. Details of the design of the RBCT have been described elsewhere [8, 10]. The analyses reported here focus on badgers culled in proactive areas of the RBCT. Proactive culling consisted of an initial cull across all accessible land in the trial area (consent was requested from landowners for culling to be performed on their land, hence badgers were only trapped on this accessible land, however, it has been shown elsewhere [14] that trapping strategies caught as many badgers per square kilometre from the inaccessible land as from the accessible land). The 10 initial culls occurred between 1998 and 2002, followed by approximately annual culls until 2005. Culling was suspended between May 2001 and January 2002 due to a nationwide epidemic of foot-and-mouth disease (FMD). Badgers were captured by cage trapping, dispatched by shooting and the carcases transferred to one of several laboratories for necropsy. Throughout this paper, year refers to ‘badger year’ measured from 1 February to 31 January as most cubs are born in February.
Necropsy
The large numbers of animals trapped during the culling operations necessitated only 15 min be devoted to the necropsy of each carcase. A standard operating procedure [15] was followed involving examination and sampling of a prescribed set of tissues. Cubs were identified on the basis of their small size and unworn teeth (see Table 1 for baseline statistics). Each carcase was examined first for the presence of trap injuries [16] and bite wounds. All bite wounds, irrespective of whether or not they were suggestive of M. bovis infection, were recorded. Throughout this paper, bite wounds will be referred to as such and the word lesions will never refer to a bite wound. Eighteen pre-specified tissue sites in the body (see Table 2) were then excised and examined for lesions suggestive of TB and throughout this paper, the word lesions only refers to those found at any of these 18 sites. Large organs were multiply incised to look for lesions. Multiple longitudinal incisions were made to the lungs at intervals of about 1 cm and the surface of the pericardial sac was examined. Both the liver and kidneys were incised in several places and the cut surfaces examined. The risk of cross-contamination of samples between badgers was addressed by using a new set of sterile instruments and a new set of clean, disposable gloves for each badger and disinfecting the down-draft table and pathologist's gown after each badger examination. The independent auditor confirmed that procedures in place would have minimized the risk of cross-contamination between animals [17]. Lesions were scored for severity as follows: 1=a single lesion; 2=2–3 lesions; 3=multiple (>3) lesions affecting parts of tissue; 4=diffuse lesions throughout the tissue. Here, we define a lesion suggestive of TB as a discrete pale consolidated area, visible on the cut surface of the tissue; a lesion may range from a few millimetres to several centimetres in diameter and may contain calcification and/or a central area of caseous necrosis. All reference to lesions in this paper is to those that conform to this definition. If a lesion was found at any of these sites, the badger was classed as lesion positive and the individual lesion was given a severity score as defined above. These 18 sites were classed as being in one of five body compartments (distinct regions of the body, see Table 2). The lungs and other thoracic tissues were classified as separate body compartments to enable detailed analyses of different components of the respiratory tract.
Table 1.
Distribution of tuberculous badgers by sex, year and the occurrence of badgers showing various degrees of pathology. Badgers are grouped by age (adults or cubs) and by the presence of bite wounds (adults only)
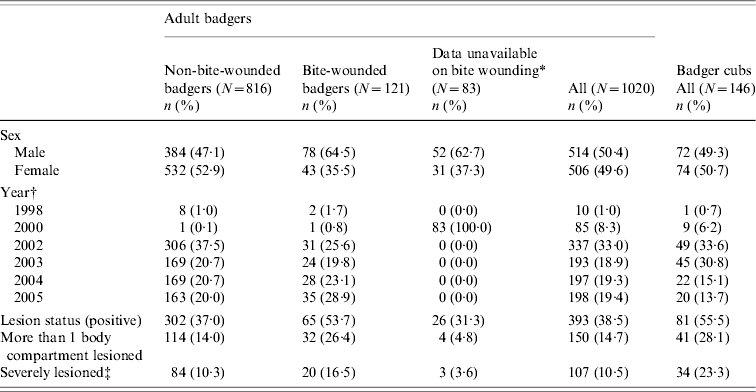
Bite wound data were not available for the majority of badgers in 2000.
All badgers culled in 1999 were stored for more than 7 days and therefore excluded. No badgers were culled in 2001 as the Randomized Badger Culling Trial (RBCT) was suspended during the foot-and-mouth epidemic. Year refers to a badger year 1 February to 31 January.
Severely lesioned has been defined as having an index value of ⩾8. Values >7·9 make up the top 25% of indices.
Table 2.
Proportion of tuberculous adult badgers with lesions and severity scores of lesions in different sites and body compartments
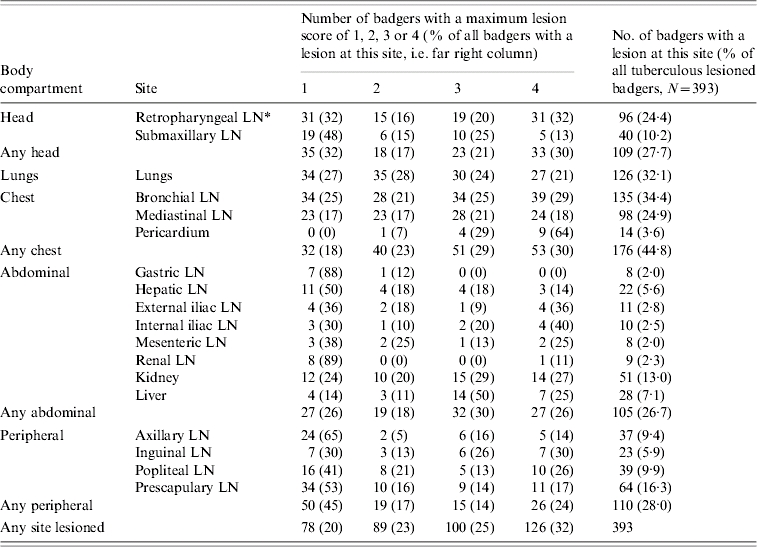
LN, Lymph node.
Bite wounds are considered separately to lesions. A bacteriological examination for the presence of M. bovis in bite wounds was not made in the present study and therefore no assumptions about the M. bovis status of bite wounds are made. Instead, for certain analyses, badgers are separated into two groups, those with and those without a bite wound at necropsy, and any differences between these two groups with regard to infection status and (for tuberculous badgers only) lesion distribution are examined.
A standard sample of lymph nodes was collected for microbiological culture [17]. This sample consisted of one half of each retropharyngeal, both bronchial, and the largest and most accessible mediastinal lymph node, as well as all lesions suggestive of TB. The tissue specimens were submitted for culture unfrozen, in a universal container with 1% cetylpyridinium chloride (CPC) in a ratio not greater than one part tissue to five parts CPC and dispatched on the day of collection for culture. If the tissues were not submitted on the day of collection, they were stored at room temperature overnight and then at 4°C thereafter. Where sufficient lesioned tissue was available, additional samples were taken for histology. Samples for histology were fixed in at least ten times their volume of 10% formol saline. At the culture laboratory, the small samples and the remaining half of the larger lesion samples were homogenized and the resultant homogenate was then used to inoculate triplicate slopes of Middlebrook's 7H11 medium and another set of triplicates was set up on a medium from a different batch of the same medium. These six tubes were incubated for up to 6 weeks and cultures checked weekly for evidence of bacterial growth. Samples from those animals that gave negative culture results were subsequently sectioned for Ziehl–Neelsen staining. Badgers were considered tuberculous if M. bovis was isolated from any sample by bacteriological culture, or if they had lesions with histology characteristic of TB containing acid-fast bacteria detected by Ziehl–Neelsen staining [5]. A lesion was considered tuberculous if M. bovis was isolated from it or on histology it contained acid-fast bacteria and in this paper all reference to lesions in tuberculous badgers is to those lesions that conformed to this definition. A full set of results for only those badgers from which M. bovis was isolated from any sample by bacteriological culture (i.e. excluding those which had lesions with histology characteristic of TB containing acid-fast bacteria detected by Ziehl–Neelsen staining) is available online as Supplementary Information.
A percentage of carcases (9·15% of total carcases) had to be stored (either chilled at 0–4°C or frozen at−20°C) for more than 7 days prior to necropsy. Ninety-eight percent of these stored carcases were in 1999 and 2000 with the remainder in 1998 and 2002. Badgers trapped between August 1999 and March 2000 had to be frozen following instructions from the Health and Safety Executive. At other times they were stored frozen when the rate at which they were culled and delivered exceeded that at which they could be examined. There is evidence that such storage may reduce the sensitivity of detecting M. bovis by culture [18]. Analyses conducted using data from proactive areas in the RBCT showed a borderline significant association between being culture negative for M. bovis and having been stored for more than 7 days [19]. As our main interest in these analyses is the pathology in tuberculous badgers and freezing is also likely to have influenced detection of lesions at necropsy, data for all carcases stored for more than 7 days were excluded from the analysis. Information on bite wounds had not been recorded for 1315 adult badgers and 223 cubs so these badgers were excluded from those analyses comparing badgers with or without bite wounds.
Since the objective of this study was to investigate the nature and extent of the visible pathology caused by M. bovis in badgers, the analyses focused on the 1166 confirmed tuberculous badgers (other than prevalence of infection which must use the total 8052 badgers – or appropriate subsets).
Statistical methods
Descriptive statistics were compiled to ascertain the proportions of tuberculous badgers with lesions and the proportions with lesions in each site and body compartment and in multiple sites. Associations between presence of lesions in the head, lungs and chest compartments were calculated. The analyses were conducted on a dataset of 1166 tuberculous badgers, comprising 146 cubs and 1020 adults. Prevalence of infection and the distribution of lesions in subsets with and without bite wounds were compared.
Three different measures of lesion severity in tuberculous badgers were considered: (i) the severity of those lesions present, expressed as a mean severity score across lesioned sites (‘average score’); (ii) the extent of lesion dissemination, expressed as the number of body compartments affected (out of five); and (iii) the extent of involvement of affected body compartments, expressed as the average number of sites per body compartment affected (max 18/max 5).
Based on the distributions, in particular the variances, of these three variables, a lesion index sensitive to plausible patterns of change was defined and calculated for each lesioned badger.
 |
For a badger with one lesioned site, this index was equal to the score at this site. The index then increased if more body compartments were lesioned and if more lesions were present in multiple sites in one body compartment. Equivalently, this index can be thought of as the total score penalized for having multiple sites per distinct body compartment.
RESULTS
Prevalence of infection and prevalence of visible lesions in tuberculous badgers
A total of 1020 (15·9%) of the 6432 adult badgers subjected to necropsy were found to be tuberculous (i.e. were culture positive or had histological lesions typical of TB containing acid-fast organisms). These 1020 comprised our dataset of adult badgers for subsequent analysis. Of these animals, 393 (38·5%), representing 6·1% of all adult badgers examined, had visible lesions confirmed as tuberculous. Of the 1620 cubs examined, 146 were tuberculous resulting in a significantly lower prevalence than in the adults (9% compared to 15·9%) although the proportion of the 146 tuberculous cubs that had visible lesions was significantly higher than for the adults (55·5% compared to 38·5%).
Distribution of lesions in tuberculous badgers
Summaries of the frequency and severity of lesions in different sites and body compartments are provided in Tables 2 and 3. Of the tuberculous animals with visible gross lesions, 78% had lesions in one or more of the head, lungs and chest compartments; 50% had lesions in the lungs and/or chest and 15% had lesions in the head only. Among tuberculous adult badgers, there were highly significant positive associations between lesion presence in the chest and head [unadjusted odds ratio (OR) 4·19, 95% confidence interval (CI) 2·74–6·40] and between chest and lungs (unadjusted OR 5·38, 95% CI 3·60–8·03). Although the association between lesion occurrences in the lungs and head was significant, it was considerably weaker (unadjusted OR 1·71, 95% CI 1·01–2·89).
Table 3.
Involvement of the head, lungs and chest in lesioned, tuberculous adult badgers
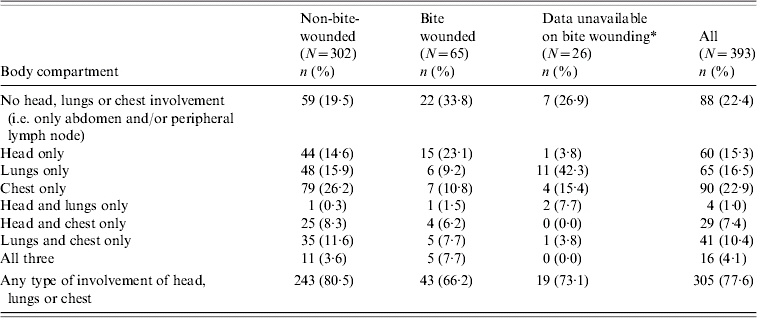
Bite-wound data were not available for the majority of badgers in 2000.
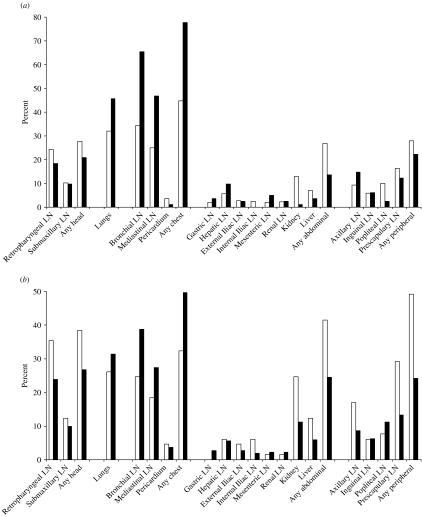
Nearly 80% of the lesioned, tuberculous cubs had lesions in the chest, followed by 46% in the lungs. These proportions are significantly higher than in the same body compartments in adults. Proportions in the head, abdominal compartment and peripheral lymph nodes of cubs were 21%, 14% and 22% respectively – all lower than in adults although only significantly lower in the abdominal compartment (see Fig. 1a).
Fig. 1.
The distribution of lesions between body compartments in tuberculous badgers. (a) Proportion of badgers with lesions by tissue site, for adult badgers and for cubs; white bars indicate adult badgers (n=393) and black bars badger cubs (n=81). (b) Proportion of tuberculous adult badgers with lesions by tissue site, for badgers with and without a bite wound at necropsy; black bars indicate badgers without a bite wound at necropsy and a lesion in at least one site (n=302) and white bars badgers with a bite wound at necropsy and a lesion in at least one site (n=65). LN, Lymph node.
Severity of pathology
The lesion scores for different sites and body compartments of tuberculous adult badgers are summarized in Table 2. Sites with lesion scores of 3 or 4, indicating the presence of multiple lesions, were found in all body compartments but the highest frequencies of such lesions were detected in the lungs and the bronchial, mediastinal and retropharyngeal lymph nodes. However, only 27 animals (7% of the lesioned tuberculous badgers) had a lung lesion score of 4, which is indicative of advanced disease, and only 57 (14·4%) had lung lesion scores of 3 or 4.
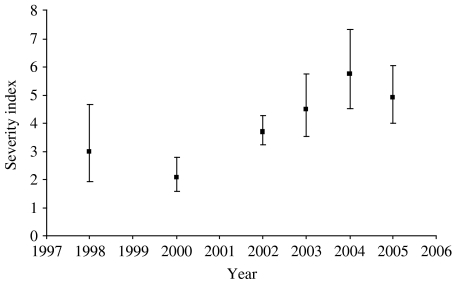
The distribution of the severity index is shown in Table 4. The majority of tuberculous badgers had a low index; 61% had an index of ⩽4 and only 10% had a score of ⩾16, ranging up to the highest of 91. Twenty-two badgers (5·6% of lesioned tuberculous badgers) had extensively disseminated disease with four or five different body compartments containing at least one site with a lesion score of 4. Thus, only a small minority of tuberculous animals had extensive and severe pathological lesions. Figure 2 shows how the severity index varied over time. While there is an increasing trend in the index over time, data were inadequate to draw any robust conclusions as to the reasons for this trend.
Table 4.
Distribution of the lesion indices, showing how individual factors of the index are represented through the distribution (only lesioned, tuberculous adult badgers included)
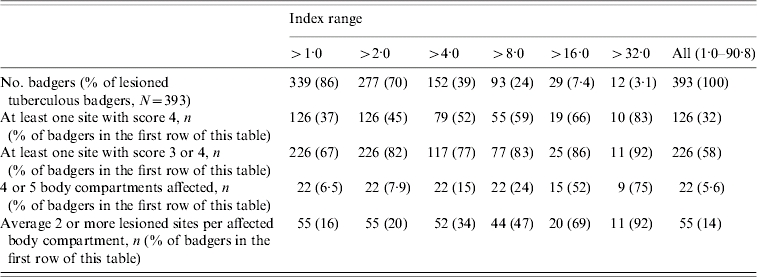
Fig. 2.
Observed severity indices: error bars give the 95% confidence limits. All badgers culled in 1999 were stored for more than 7 days and therefore excluded. There are no data in 2001 as proactive culling in the Randomized Badger Culling Trial (RBCT) was temporarily suspended due to the foot-and-mouth disease epidemic that occurred between February and November 2001. Year refers to the badger year from 1 February to 31 January.
Prevalence of infection and distribution of lesions in animals with and without bite wounds
Bite wounds were found in 272 (5·3%) of the 5117 adult badgers for which the presence or absence of bite wounds was recorded. Of the 4845 adult badgers that did not have a bite wound, 816 (16·8%) were found to be tuberculous compared to 121 (44·5%) of the 272 badgers with detectable bite wounds. An unadjusted odds ratio showed that a tuberculous adult badger was four times more likely to also have had a bite wound evident at necropsy than a non-tuberculous adult badger (OR 3·96, 95% CI 3·08–5·08). When a binary variable indicating presence or absence of a bite wound was added to a model already shown to predict prevalence of infection based on the same data [19], a strong association was observed. Tuberculous badgers were 4·23 (95% CI 3·24–5·52) times as likely to have a bite wound as non-tuberculous badgers (P<0·0001). Of cubs with data on bite wounds, 18 (1·3%) had a bite wound at necropsy. Bite wounds in cubs were similarly positively associated with a detectable infection (unadjusted OR 6·11, 95% CI 2·33–16·04). More detailed analyses on bite-wounded cubs were not possible due to small numbers.
The proportion of tuberculous adult badgers with detectable lesions also differed when split into badgers with bite wounds (53·7% had lesions) and those without (37·0% had lesions). Similarly, the proportion of tuberculous badgers with lesions in multiple body compartments was consistently higher among badgers with bite wounds – 26% of bite-wounded, tuberculous badgers had lesions in more than one body compartment compared to 14% of tuberculous badgers with no bite wound (see Tables 1 and 5).
Table 5.
Number of body compartments affected with lesions for badgers with and without a bite wound (only tuberculous adult badgers are included)
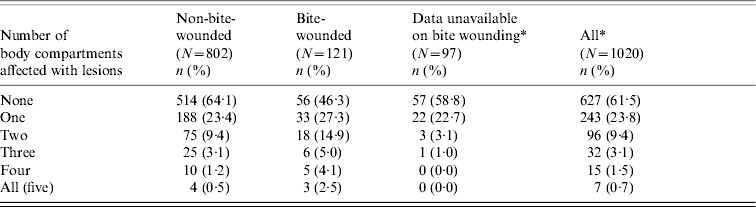
Bite-wound data were not available for the majority of badgers in 2000.
The locations where lesions were predominantly found in tuberculous adult badgers differed between animals that had bite wounds and those that did not. Lesioned tuberculous badgers with no bite wound had a higher prevalence of lesions in the lungs and chest compared to badgers with a bite wound (lungs: 31% compared to 26%; chest: 50% compared to 32%). Conversely, lesioned tuberculous badgers with bite wounds had a higher prevalence of lesions in the head, abdomen and peripheral lymph nodes (38% vs. 27%, 42% vs. 25% and 49% vs. 24%, respectively; see Fig. 1b).
DISCUSSION
The results of this study provide, for the first time, quantitative information on the prevalence of infection and severity of pathology in a large sample from a population of badgers infected with M. bovis. About 16% of the adult badgers were found to be tuberculous, of which about 39% (6·1% of all adult badgers) had visible pathological lesions (as detected at necropsy). However, only a very small proportion of the lesioned tuberculous animals had disseminated pathology that might have resulted in clinical disease.
The detection of large numbers of tuberculous badgers with no visible lesions has been a consistent finding in a number of earlier studies, although the percentages of non-lesioned infected animals found in these studies varied, ranging from 28% to 72% [3–5]. It is worth emphasizing, however, that both the identification of infected animals and the detection of lesions suggestive of TB are dependent on the methods employed. Thus, in some of these studies, animals were subjected to very detailed necropsy, whereas in the present study only limited time (15 min per carcase) was devoted to examination of each animal and therefore some lesions may have been missed, particularly small lesions in larger organs such as the lungs, liver and kidneys.
Recent unpublished studies have also shown that more rigorous bacteriological examination of tissues from a random sample of RBCT badgers resulted in the detection of almost twice as many infected animals as were revealed by the standard culture protocol (T. R. Crawshaw, I. B. Griffiths and R. S. Clifton-Hadley, unpublished observations). In this more detailed protocol, samples for culture and histopathology were collected from several additional tissues, regardless of whether lesions suggestive of TB were found. Twice the number of culture tubes (12 rather than six) were used and were incubated for twice as long (12 weeks rather than 6 weeks). The results of this study showed that the standard sampling and culture protocol used in the trial detected only 55% of the animals demonstrated to be tuberculous by this enhanced protocol. It was, however, noted that very few additional lesioned badgers were found and most of these additional tuberculous badgers had no lesions at all. These findings suggest that the methodologies employed in the trial did not substantially compromise the ability to detect lesions in tuberculous animals. However, the culture-sensitivity results indicate that the true prevalence of infection in the RBCT badgers was almost certainly substantially higher than the 16% reported here; but since these additional infected animals are likely to have less severe pathology (T R. Crawshaw, I. B. Griffiths and R. S. Clifton-Hadley, unpublished observations), the proportion of tuberculous badgers that show evidence of gross pathology is almost certainly lower than the observed value of 39%.
Previous studies have demonstrated the presence of microscopic lesions typical of TB in animals that showed no gross pathology at necropsy; by careful scrutiny of thin slices of fixed tissue in combination with histological examination of selected sites, Gallagher et al. [20] were able to detect such lesions in 13 out of 15 culture-positive badgers with no gross lesions. The authors argued that infection in these animals was contained, implying that the animals were not infectious. However, there is no direct evidence to support this conclusion and, even if only a small proportion of them are able to transmit infection, quantitatively they would represent an important source of infection for transmission. This would be consistent with experimental findings in cattle that showed excretion of M. bovis in the absence of visible gross lesions [21].
A striking finding in the present study was the small proportion of tuberculous animals that exhibited severe, disseminated pathology. This is in contrast to the findings of some previous studies [3, 13]. For example, the study conducted by Gallagher & Nelson [3] reported miliary TB (i.e. disseminated pathology) in about 16% of a sample of more than 200 M. bovis-infected badgers, comprising animals removed from TB-affected farms plus road-traffic deaths. The selected nature of the samples of animals examined in these previous studies, and in some cases the small numbers of animals included, may account for differences in values obtained for the proportions of animals showing visible lesions or severe pathology. RBCT findings have shown that the prevalence of infection can differ spatially both at the level of badger social groups and region [19] suggesting that samples of animals drawn from a small number of groups may not be representative of the wider population. We acknowledge three potential sources of bias in the sampling method. First, the badgers in our study were cage trapped prior to culling and there is evidence that some badgers are ‘trap-shy’ and can never be caught with this method [22] and hence do not contribute to our sample. However, Tuyttens et al. [22] did not find evidence that the TB status of badgers in their study had any impact on their likelihood of being trap-shy and so it seems unlikely that this bias would have affected our results. Second, the trial areas in the RBCT were chosen to centre on TB ‘hotspots’ and as such, the badgers in our sample may have a higher prevalence of infection than others in the United Kingdom. Third, many of the badgers in our study had lived in an area which was subject to culling (i.e. many of the adult badgers were caught on a cull later than the initial cull) and for this reason had a higher prevalence of infection (as shown by Woodroffe et al. [19] using the same set of badgers). However, it is unclear whether the fact that the group had a higher prevalence of infection would have had any impact on the severity of disease or distribution of lesions among tuberculous badgers. For this reason, the sample of badgers in our current study may not be representative of the wider badger population.
The term ‘super excretor’ has been used to describe those badgers that, in longitudinal epidemiological studies, were found to shed M. bovis in sputum, urine or faeces on at least two occasions [23]. Such animals were shown to have a significantly higher mortality rate, indicating that they included those animals that go on to develop advanced disease. In separate studies, Gallagher & Clifton-Hadley [5] have shown that the lesions in animals with severe disseminated pathology can contain large numbers of M. bovis organisms. Nolan & Wilesmith [4] have also presented evidence that tissues from animals with the most severe pathology tended to contain the largest number of M. bovis organisms. It has been hypothesized that these animals with severe pathology and high bacterial loads have a particularly important role in transmitting M. bovis infection to cattle [5]. However, in the present study, only a small proportion of infected animals were found to have severe disseminated pathology; thus, there were only 27 tuberculous badgers (0·4% of all adult badgers) with a maximum lung lesion score of 4 and only 57 tuberculous animals (0·9%) had lung lesions scores of 3 or 4. These animals were spread evenly over time and between trial areas. Hence, although these badgers could provide a significant individual source of infection, they are unlikely to be responsible for the majority of transmission of infection to other badgers or to cattle and it appears more likely that the relatively larger proportion of badgers that have less severe pathology are additionally responsible for spread of infection.
The distribution of lesions in tuberculous badgers was broadly similar to that reported in previous studies [5, 13, 24]. The observed predominance of lesions in the thorax (lungs and chest compartments) and the head is consistent with the evidence that M. bovis is transmitted mainly by the respiratory route. As expected, there was a strong association between the presence of lesions in the lungs and in the mediastinal and bronchial lymph nodes, which drain the lower respiratory tract. There was also an association between the presence of lesions in the head and chest lymph nodes but a weaker association between head lymph-node lesions and lesions in the lung. The latter finding may reflect different routes of infection, with infection by inhalation of large aerosol particles or by the oral route giving rise to pathology in the lymph nodes in the head, and inhalation of small aerosol particles resulting in lesions focused in the lungs and associated nodes. The involvement of upper and lower respiratory routes of infection has also been proposed to account for different patterns of pathology in naturally infected cattle, and is supported by observations on the distribution of lesions in cattle experimentally infected by the intranasal [21] or intra-tracheal route [12].
Analysis of badgers with recorded bite wounds (~5% of adults) at necropsy revealed that this subset of animals had a significantly higher prevalence of detectable infection. It is possible, however, that bite wounds that have been infected with M. bovis (as some of these probably are) would take longer to heal and would therefore be more likely to be seen at necropsy than uninfected bite wounds that would have healed more quickly. Such an effect would increase the positive association found between infection and bite wounds but would not have had any effect on analysis on the subset of tuberculous badgers only. Although information on the presence of M. bovis in bite wound tissues was not available for animals in the present study, the presence of lesions containing M. bovis in bite wounds of infected badgers has been reported previously [5, 13]. Among tuberculous badgers, there was a higher prevalence of visible lesions and more widespread lesions compared to tuberculous animals with no bite wounds. Differences in the distribution of lesions in these animals indicate that a proportion of them are likely to have become infected via the bite wounds. A previous study has also shown that bites are most commonly observed in the rump, followed by the head, with the torso being least affected [25]. The higher prevalence of lesions in peripheral and head tissues is consistent with dissemination of infection from skin lesions at these sites to the draining lymph nodes; moreover the increased numbers of tissues affected and the increased abdominal involvement in animals with bite wounds may reflect a higher likelihood of haematogenous spread of infection to visceral organs in these animals. The distribution of lesions in tuberculous cubs, which exhibited greater involvement of the respiratory tract and less involvement of peripheral and abdominal tissues, is also consistent with detection of fewer bite wounds and hence less infection via this route in this age group. Because data were not available on the presence of M. bovis in the bite wound, the conclusions are tentatively inferred from the patterns seen in the data. Although the potential for infection via bite wounds probably accounts for only a small proportion of the badger infections, this route of infection may have a significant role in transmission of infection between badger social groups, since there is evidence that bite wounds in badgers are frequently acquired during aggressive encounters between badgers from different social groups [26].
In conclusion, our analyses of necropsy data from 1166 tuberculous badgers have yielded valuable insights into the disease caused by M. bovis in badgers and how it is transmitted. The findings confirm previous observations that a majority of tuberculous badgers have no grossly visible lesions. However, the finding that only a small number and proportion of tuberculous badgers have severe and extensive pathology contrasts with the results of a number of other studies. This implies that severely diseased animals are unlikely to be the sole source of infection for transmission from badgers to the cattle population and it appears likely that the relatively larger proportion of badgers that have less severe pathology are additionally responsible for spread of infection. The higher prevalence of pathology and different distribution of lesions in tuberculous badgers with recorded bite wounds indicate that transmission may occur via bite wounds and that such animals may have a higher likelihood of developing overt pathology. However, the predominant involvement of the respiratory tract in most animals indicates that the main transmission route is respiratory, with patterns of lesions focused mainly in the head lymph nodes or lungs and associated nodes suggesting acquisition of infection via the upper or lower respiratory tract respectively.
ACKNOWLEDGEMENTS
This study was funded and implemented by the Department of Environment, Food and Rural Affairs (Defra). We gratefully acknowledge the work of the Defra Wildlife Unit which conducted all RBCT fieldwork, and the Veterinary Laboratories Agency and Central Science Laboratory which performed all diagnostic work. We also thank the many farmers and land occupiers who allowed data collection on their land. We thank three anonymous reviewers for their helpful comments.
NOTE
Supplementary information accompanies this paper on the Journal's website (http://journals.cambridge.org).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Muirhead RH, Gallagher J. Tuberculosis in wild badgers in Gloucestershire – Epidemiology. Veterinary Record. 1974;95:552–555. doi: 10.1136/vr.95.24.552. [DOI] [PubMed] [Google Scholar]
- 2.Cheeseman CL, Wilesmith JW, Stuart FA. Tuberculosis: the disease and its epidemiology in the badger, a review. Epidemiology & Infection. 1989;103:113–125. doi: 10.1017/s0950268800030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher J, Nelson J. Cause of ill health and natural death in badgers in Gloucestershire. Veterinary Record. 1979;105:546–551. [PubMed] [Google Scholar]
- 4.Nolan A, Wilesmith JW. Tuberculosis in badgers (Meles meles) Veterinary Microbiology. 1994;40:179–191. doi: 10.1016/0378-1135(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher J, Clifton-Hadley RS. Tuberculosis in badgers; a review of the disease and its significance for other animals. Research in Veterinary Science. 2000;69:203–217. doi: 10.1053/rvsc.2000.0422. [DOI] [PubMed] [Google Scholar]
- 6.Clifton-Hadley RS, Wilesmith JW, Stuart FA. Mycobacterium bovis in the European badger (Meles meles): epidemiological findings in tuberculous badgers from a naturally infected population. Epidemiology & Infection. 1993;111:9–19. doi: 10.1017/s0950268800056624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krebs JR, Bovine Tuberculosis in Cattle and Badgers. London: MAFF Publications; PB3423: 1997. [Google Scholar]
- 8.Defra. 2007. Bovine TB: The Scientific Evidence. Final Report of the Independent Scientific Report on Cattle TB,
- 9.Donnelly CA. et al. Impact of localized badger culling on tuberculosis incidence in British cattle. Nature. 2003;426:834–837. doi: 10.1038/nature02192. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly CA. et al. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature. 2006;439:843–846. doi: 10.1038/nature04454. [DOI] [PubMed] [Google Scholar]
- 11.McFadyean J. What is the common method of infection in tuberculosis? Journal of Comparative Pathology. 1910;23:289–303. [Google Scholar]
- 12.Dean GS. et al. Minimum infective dose of Mycobacterium bovis in cattle. Infection and Immunity. 2005;73:6467–6471. doi: 10.1128/IAI.73.10.6467-6471.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher J, Muirhead RH, Burn KJ. Tuberculosis in wild badgers (Meles meles) in Gloucestershire: pathology. Veterinary Record. 1976;98:9–14. doi: 10.1136/vr.98.1.9. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly CA. et al. Impacts of widespread badger culling on cattle tuberculosis: concluding analyses from a large-scale field trial. International Journal of Infectious Diseases. 2007;11:300–308. doi: 10.1016/j.ijid.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Hall G. Defra; 2004. . Report of the independent auditor on the badger post mortem procedures used in the Randomised Badger Culling Trial and Defra's reponse. [Google Scholar]
- 16.Woodroffe R. et al. Welfare of badgers (Meles meles) subjected to culling: patterns of trap-related injury. Animal Welfare. 2005;14:11–17. [Google Scholar]
- 17.Corbel M2004. . Report of the independent audit of bacteriological culture carried out on tissue samples collected during the Randomised Badger Culling Trial and Defra's response,
- 18.Defra . The effect on viability of Mycobacterium bovis of freezing samples prior to cultural testing, 2005
- 19.Woodroffe R. et al. Culling and cattle controls influence tuberculosis risk for badgers. Proceedings of the National Academy of Sciences USA. 2006;103:14713–14717. doi: 10.1073/pnas.0606251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher J. et al. Role of infected, non-diseased badgers in the pathogenesis of tuberculosis in the badger. Veterinary Record. 1998;142:710–714. doi: 10.1136/vr.142.26.710. [DOI] [PubMed] [Google Scholar]
- 21.McCorry T. et al. Shedding of Mycobacterium bovis in the nasal mucus of cattle infected experimentally with tuberculosis by the intranasal and intratracheal routes. Veterinary Record. 2005;157:613–618. doi: 10.1136/vr.157.20.613. [DOI] [PubMed] [Google Scholar]
- 22.Tuyttens FAM. et al. Differences in trappability of European badgers Meles meles in three populations in England. Journal of Applied Ecology. 1999;36:1051–1062. [Google Scholar]
- 23.Wilkinson D. et al. The effects of bovine tuberculosis (Mycobacterium bovis) on mortality in a badger (Meles meles) population in England. Journal of Zoology. 2000;250:389–395. [Google Scholar]
- 24.Nolan A . An investigation into the development of specific antibody responses of badgers (Meles meles) to infection with Mycobacterium bovis with reference to the pathogenesis and epidemiology of disease (Ph.D. thesis). Brunel University, 1991 [Google Scholar]
- 25.Delahay RJ. et al. Demographic correlates of bite wounding in Eurasian badgers, Meles meles L., in stable and perturbed populations. Animal Behaviour. 2006;71:1047–1055. [Google Scholar]
- 26.Kruuk H. The Social Badger. Oxford: Oxford University Press; 1989. [Google Scholar]