SUMMARY
Recently, a changing pattern of hepatitis A epidemiology has been reported in the Indian population indicating a rise in the rate of hepatitis A infection among adults. The study's objective was to assess anti-HAV prevalence in voluntary blood donors from middle and high socioeconomic strata. Serum samples collected from voluntary blood donors from Pune city and its suburbs in the years 2002 and 2004–2005 were tested for anti-HAV IgG antibodies. Serum samples collected during 2004–2005 were examined for anti-HAV IgM antibodies. Positive samples were tested for HAV-RNA. Agewise anti-HAV positivity was significantly low in adults aged 18–25 years (90·4%) compared to those aged >25 years (97·4%) (P<0·01). A decline in anti-HAV prevalence was significant in 2004–2005 compared to that in 2002 (96·5% vs. 92·1%) (P<0·01). Overall, in both adult age groups, the proportion of anti-HAV positivity was remarkably low in the high socioeconomic group (HSG) (88·96%) compared to that of the middle socioeconomic group (MSG) (95·86%) (P<0·01). Anti-HAV IgM positivity was not significant (~1%), however, presence of HAV-RNA in one of the samples indicated the possibility of horizontal transmission of HAV. Increase in seronegativity to HAV in HSG implicates a rise in the susceptible pool and indicates the need for vaccination against hepatitis A.
Hepatitis A is an enterically transmitted disease caused by hepatitis A virus (HAV). The disease has a wide distribution throughout the world. The severity of the disease is related to the age at infection [1]. The rate of infection markedly depends on the socioeconomic development of the region or country [2, 3]. In the developed countries of western Europe and North America, and in Japan and Australia, the proportion of anti-HAV seropositive persons is low in childhood. This increases during adolescence and early adulthood, and reaches a high level by late adulthood. In most developing countries in Africa and Asia, antibodies usually appear early in life and remain detectable in adulthood. However, several previous reports have documented the changing trend of hepatitis A epidemiology in developing countries [4–8].
In India, hepatitis A is mainly encountered by the paediatric population [9]. Occurrence of outbreaks of hepatitis A have been also reported [10–12]. Recent surveillance studies carried out in various communities in India document declining anti-HAV prevalence and its association with improved socioeconomic status and the quality of water supply, and an increase in education level and personal hygiene [13–16]. However, this has increased the risk of HAV infection in the adult population [12, 17]. The present study was conducted to assess the prevalence of anti-HAV antibodies in voluntary blood donors who represent the middle and high socioeconomic status population of adults in India.
The subjects under study included healthy voluntary blood donors from Pune city and the suburbs of Pune district. These comprised 991 males and 154 females aged 18–50 years. Informed consent was obtained from all individuals. A preset questionnaire inclusive of jaundice, vaccination, source of water supply, education level and monthly income, was duly completed for each subject. Based on monthly income, the study population was classified into middle, and high socioeconomic status [18]. The test specimens consisted of 724 and 421 blood samples collected respectively in 2002 and 2004–2005 during blood donation camps. All serum samples were stored at −20° C until tested.
Serum samples collected during 2002 and 2004–2005 were tested by ELISA for anti-HAV IgG antibodies, a marker of past infection. The 2004–2005 samples were also tested by ELISA for anti-HAV IgM antibodies, a marker of recent infection [19–21]. RT–PCR was performed according to the method described previously [22] on anti-HAV IgM-positive serum samples to detect HAV-RNA using primers from the RNA polymerase region of the HAV genome.
The differences between the proportions of seropositivity among different groups of donors were compared using the χ2 test. For small samples, Fisher's exact test was used.
A total of 1145 serum samples collected during 2002 and 2004–2005 were tested for anti-HAV antibody. The agewise prevalence of anti-HAV in blood donors during the years 2002 and 2004–2005 is shown in the Table. Anti-HAV positivity was significantly low in adults aged 18–25 years compared to that in the >25 years age group (P<0·01). Overall, 96·5% (699/724) of the donors in 2002 were found to be anti-HAV positive compared to 92·16% (388/421) in 2004–2005. A highly significant decline in anti-HAV prevalence was noted in 2004–2005 (P<0·01). The decrease in anti-HAV prevalence was not uniform in the two age groups investigated in the study. In 2004–2005, anti-HAV positivity was significantly different in the >25 years age group (P<0·01) from that of 2002 while for these years it was similar in the 18–25 years age group (P>0·05).
Table.
Agewise anti-HAV prevalence in voluntary blood donors in the years 2002 and 2004–2005
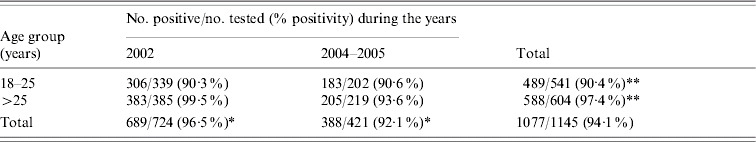
P<0·01 (2002 vs. 2004–2005)
P<0·01 (age group 18–25 vs.>25 years).
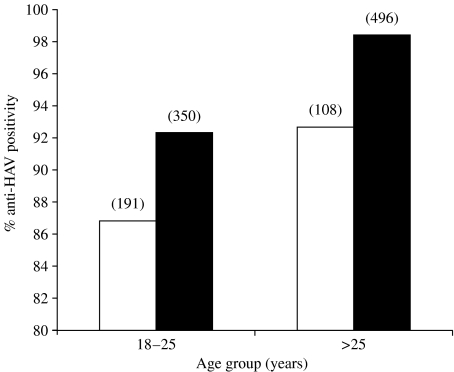
Since the blood donors of the study belonged to high and middle socioeconomic groups (HSG and MSG), the seroprevalence analysis was performed on the basis of their socioeconomic status. Anti-HAV positivity among adults of both age groups (18–25 and >25 years) was significantly low in the high socioeconomic population (86·91%, 92·59%) compared to the corresponding age groups from the middle socioeconomic population (92·29%, 98·39%) (P<0·01) (Fig.). On the whole, HSG showed a significantly reduced anti-HAV prevalence compared to MSG (88·96% vs. 95·86%) (P<0·01).
Fig.
Anti-HAV prevalence in voluntary blood donors based on socioeconomic status. Figures in parentheses indicate number of samples examined. □, High socioeconomic group; ■, Middle socioeconomic group.
Four of 421 serum samples showed positivity to anti-HAV IgM antibodies. These four samples were processed further to detect the presence of HAV-RNA by RT–PCR. One of the samples testing positive was confirmed for HAV genome by sequencing the amplicon.
The present study detects hepatitis A antibodies in voluntary donors. All donors were negative for hepatitis B and human immunodeficiency virus infections. In 2004–2005 the extent of exposure to HAV was lower compared to that of 2002 (P<0·01). These observations are similar to the recent reports documented from different parts of India [13, 16] and suggest intermediate endemicity of hepatitis A in this region. Overall, anti-HAV positivity was low in the 18–25 years age group, however, the decline in seropositivity to HAV among the donors during 2004–2005 had a significant proportion aged >25 years (Table). This may be due to the proportion of MSG (30·6%) and HSG (16·7%) of the 18–25 years age group in the present study. Alternatively, this may provide an assumption for variable improvements in hygienic and socioeconomic conditions in the Indian population. Prevalence of hepatitis A antibodies in HSG donors compared to MSG was remarkably low in both the age groups (P<0·01) as well as at two different time points studied. These observations correlate with those reported previously from India [7, 15–17].
The serum samples collected from healthy donors were expected to be negative for anti-HAV IgM. However, four donors tested positive for IgM antibodies. Although, anti-HAV IgM prevalence was very low, it implicated recent HAV infection at the subclinical level. One of the samples, collected from a 20-year-old MSG female, tested positive for HAV-RNA and was confirmed as having a 3D polymerase sequence of the HAV genome. These data suggest the possibility of horizontal transmission of HAV by the parenteral route as documented elsewhere [23–25].
Overall, increase in seronegativity in the high socioeconomic population implicates a rise in susceptibility to HAV thereby identifying the target population for vaccination against hepatitis A.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the cooperation extended by the staff of Blood Bank, Inlaks and Budharani Hospital, Pune. Thanks are due to Shri. Rajesh Kannalu and Shri. Prakash Jawalkar for technical assistance.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.WHO. Hepatitis A vaccines. Weekly Epidemiological Record. 2000;75:38–44. [Google Scholar]
- 2.Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiology and Infection. 2004;132:1005–1022. doi: 10.1017/s0950268804002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell BP et al. Hepatitis A virus infection in United States: serologic results from the Third National Health and Nutrition Examination Survey. Vaccine. 2005;23:5798–5806. doi: 10.1016/j.vaccine.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 4.Innis BL et al. The declining transmission of hepatitis A in Thailand. Journal of Infectious Diseases. 1991;163:989–995. doi: 10.1093/infdis/163.5.989. [DOI] [PubMed] [Google Scholar]
- 5.Hong-Yuan H et al. Changing seroepidemiology of Hepatitis A virus infection in Taiwan. Journal of Medical Virology. 1985;17:297–301. doi: 10.1002/jmv.1890170402. [DOI] [PubMed] [Google Scholar]
- 6.Poovorawan Y et al. The declining pattern of seroepidemiology of hepatitis A virus infection among adolescents in Bangkok, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 1997;28:154–157. [PubMed] [Google Scholar]
- 7.Dhawan PS et al. Seroprevalence of hepatitis A virus in Mumbai and immunogenicity and safety of hepatitis A vaccine. Indian Journal of Gastroenterology. 1998;17:16–18. [PubMed] [Google Scholar]
- 8.Chitambar SD et al. Prevalence of hepatitis A antibodies in Western Indian population: changing pattern. Southeast Asian Journal of Tropical Medicine and Public Health. 1999;30:273–276. [PubMed] [Google Scholar]
- 9.Arankalle VA et al. Age specific prevalence of antibodies to hepatitis A and E viruses in Pune, India 1982 and 1992. Journal of Infectious Diseases. 1995;171:447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 10.Chitambar SD et al. Sensitive ELISA tests for detection of anti hepatitis A virus antibodies. Serodiagnosis and Immunotherapy in Infectious Disease. 1996;8:63–65. [Google Scholar]
- 11.Patnaik SK et al. An outbreak of viral hepatitis A in Ibrahimpatanam town of Andhra Pradesh. Journal of Communicable Disease. 1995;27:118–119. [PubMed] [Google Scholar]
- 12.Arankalle VA et al. Molecular charecteriazation of hepatitis A virus from a large outbreak from Kerala, India. Indian Journal of Medical Research. 2006;123:760–769. [PubMed] [Google Scholar]
- 13.Das K et al. The changing epidemiological pattern of hepatitis A in an urban population of India: emergence of a trend similar to the europian countries. European Journal of Epidemiology. 2000;16:507–510. doi: 10.1023/a:1007628021661. [DOI] [PubMed] [Google Scholar]
- 14.Mall ML et al. Seroepidemiology of hepatitis A infection in India: changing pattern. Indian Journal of Gastroenterology. 2001;20:132–135. [PubMed] [Google Scholar]
- 15.Arankalle VA et al. Changing epidemiology of hepatitis A and E in urban and rural India (1982–1998) Journal of Viral Hepatitis. 2001;8:293–303. doi: 10.1046/j.1365-2893.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 16.Jindal M et al. Serological study of hepatitis A virus infection amongst the students of medical college in Delhi and evaluation of the need of vaccination. Indian Journal of Medical Research. 2002;115:1–4. [PubMed] [Google Scholar]
- 17.Hussain Z et al. Increasing trend of acute hepatitis A in north India: Need for identification of high-risk population for vaccination. Journal of Gastroenterology and Hepatology. 2006;21:689–693. doi: 10.1111/j.1440-1746.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal OP et al. A new instrument (scale) for measuring the socioeconomic status of a family: preliminary study. Indian Journal of Community Medicine. 2005;30:111–112. [Google Scholar]
- 19.Chitambar SD et al. Indigenous anti hepatitis A virus IgM capture ELISA for the diagnosis of hepatitis A. Indian Journal of Medical Research. 1994;99:243–251. [PubMed] [Google Scholar]
- 20.Chitambar SD et al. Hepatitis A in day care center. Indian Journal of Pediatrics. 1996;63:781–783. doi: 10.1007/BF02730929. [DOI] [PubMed] [Google Scholar]
- 21.Joshi MS et al. Evaluation of urine as a clinical specimen for diagnosis of hepatitis A. Clinical and Diagnostic Laboratory Immunology. 2002;9:840–845. doi: 10.1128/CDLI.9.4.840-845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitambar SD et al. Fecal shedding of hepatitis A virus in Indian patients with hepatitis A and in experimentally infected Rhesus monkey. Hepatology Research. 2001;19:237–246. doi: 10.1016/s1386-6346(00)00104-2. [DOI] [PubMed] [Google Scholar]
- 23.Meyers JD et al. Parenterally transmitted hepatitis A associated with platelet transfusions: epidemiologic study of an outbreak in a marrow transplantation center. Annals of Internal Medicine. 1974;81:145–151. doi: 10.7326/0003-4819-81-2-145. [DOI] [PubMed] [Google Scholar]
- 24.Noble RC et al. Posttransfusion hepatitis A in a neonatal intensive care unit. Journal of the American Medical Association. 1984;252:2711–2715. [PubMed] [Google Scholar]
- 25.Arankalle VA, Chobe LP. Hepatitis E virus: can it be transmitted parenterally? Journal of Viral Hepatitis. 1999;6:161–164. doi: 10.1046/j.1365-2893.1999.00141.x. [DOI] [PubMed] [Google Scholar]