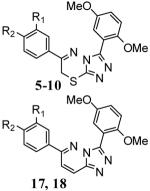
Table 1.
PDE4A inhibition by compounds 5-10, 17 and 18.
| Analogue # | R1 | R2 | PDE4A IC50 (nM) |
|
|---|---|---|---|---|
|
5 | −OCH3 | −OCH3 | 6.7 ± 0.4 |
| 6 | −OCypent | −OCH3 | 13 ± 0.8 | |
| 7 | −OCH2Cyprop | −OCH3 | 6.1 ± 0.9 | |
| 8 | −OCH2Cyprop | −OCHF2 | 11 ± 0.7 | |
| 9 | −O(3-THF)[rac] | −OCH3 | 3.4 ± 0.4 | |
| 10 | −O(3-THF)[R] | −OCH3 | 3.0 ± 0.2 | |
| 17 | −O(3-THF)[S] | −OCH3 | 7.3 ± 3.8 | |
| 18 | −O(3-THF)[R] | −OCH3 | 1.5 ± 0.7 |
data represents the results from three seperate experiments (SD provided). Definitions: OCH3 = methoxy, OCypent = cyclopentyloxy, OCH2Cyprop = cyclopropylmethoxy, OCHF2 = 2-difluoromethoxy, O(3-THF) = O-3-tetrahydrofuranyl [racemic, R or S enantiomers].
