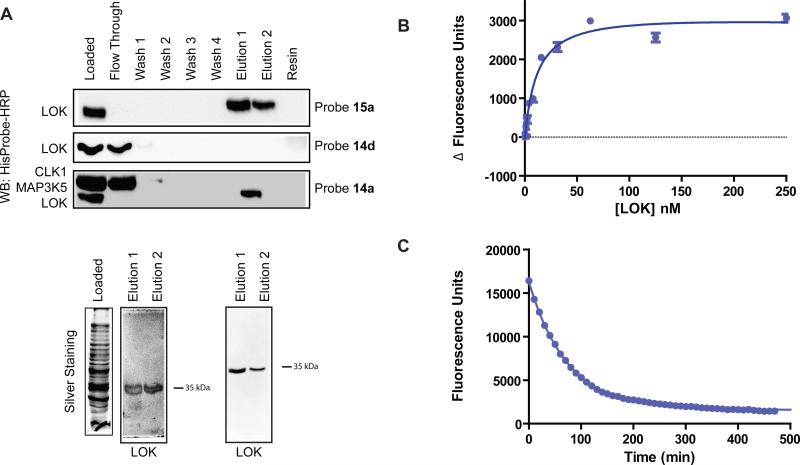
Figure 5.
The catalytic domain of LOK behaves like a kinase that can adopt the DFG-out conformation. (A) Enrichment of the catalytic domain of LOK from E. coli lysates with affinity resins 14a, 15a or control matrix 14d. Top panel: Purified His6-LOK (4.5 μg) was added to E. coli lysate (600 μg) and subjected to standard enrichment conditions with affinity matrix 15a. LOK was detected with HisProbe-HRP (Pierce). Middle panel: Purified His6-LOK (4.5 μg) was added to E. coli lysate (600 μg) and subjected to standard enrichment conditions with control matrix 14d. LOK was detected with HisProbe-HRP. Bottom panel: Purified His6-LOK (4 μg), His6-CLK1 (10 μg) and His6-MAP3K5 (10 μg) were added to E. coli lysate (500 μg) and subjected to standard enrichment conditions with affinity matrix 14a. All fractions were subjected to SDS-PAGE and kinases were detected with HisProbe-HRP (Pierce). Bottom gels: Elutions 1 and 2 from the enrichment experiments performed in the top and bottom panels above. Samples were subjected to SDS-PAGE and silver stained. (B) Change in fluorescence observed with an increasing concentration of kinase LOK in the presence of 5 nM of fluorescent probe 4a. Kd = 12 ± 1 nM. Value shown is the average of three assays ± SEM. (C) Determination of the LOK-4a complex dissociative half life (t1/2). For LOK-4a t1/2 = 51 minutes and koff = 2.3 × 10-5 s-1. Assays were run in triplicate.