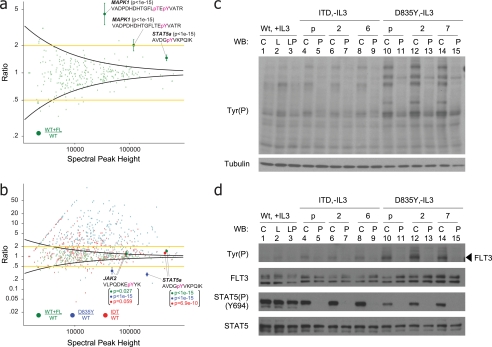
Fig. 3.
a, identification of phosphorylation regulated by WT FLT3 in cells cultured under normal serum conditions. Addition of exogenous FL increases STAT5a phosphorylation (Tyr-694) by only 1.4-fold relative to unstimulated WT FLT3. Yellow lines represent a global 2-fold threshold. Intensity-based variance function, derived from an analysis of technical replicates (black curve and Fig. 2), yields a statistically significant p value (<10e−22) and 95% confidence interval for regulation of Tyr(P)-694 on STAT5a. p values and 95% confidence intervals for phosphorylated peptides from MAPK1 are also shown. b, relative tyrosine phosphorylation levels of WT FLT3 + FL (green), FLT3-ITD (red), and FLT3-D835Y (blue), each normalized against unstimulated WT FLT3. Tyrosine phosphorylation on STAT5a (Tyr-694) and JAK2 (Tyr-1007) is coordinately up-regulated by both FLT3-ITD and stimulated WT FLT3. In contrast, phosphorylation on these sites is diminished in the context of FLT3-D835Y signaling. c, immunoblot of multiple BaF3 clones with anti-Tyr(P) antibody (specific clone or polyclonal) harboring WT FLT3 (lanes 1–3), FLT3-ITD (lanes 4–9), and FLT3-D835Y (lanes 10–15) confirmed data in b that the point mutant exhibited higher kinase activity as compared with WT FLT3 or FLT3-ITD. C, control (no stimulation); L, ligand stimulation at 50 ng/ml for 30 min; P, treatment with PKC412 at 100 nm for 30 min; p, polyclonal (otherwise specific clone designation is listed). d, immunoblot of cells in c with antibodies against phosphotyrosine, total FLT3, phospho-STAT5 (Tyr(P)-694), and total STAT5. Despite the higher constitutive phosphorylation observed for the point mutant, downstream signaling on STAT5 (Tyr(P)-694) is significantly diminished as compared with WT FLT3 and FLT3-ITD. The legend is the same as in c. pT, phosphothreonine; pY, phosphotyrosine; WB, Western blot.