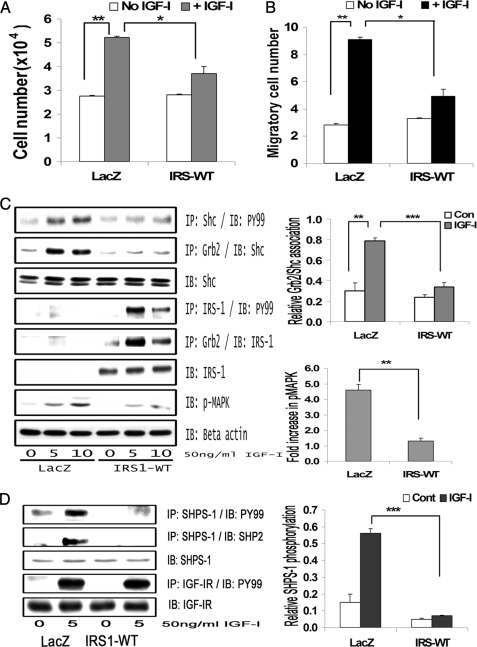
FIGURE 2.
IRS-1 overexpression impairs IGF-I-mediated SHPS-1 phosphorylation and subsequent SHPS-1 complex assembly, leading to reduction in phosphorylation of MAPK and cellular proliferation. A, LacZ and IRS-WT cells were plated (3 × 104 cells) in DMEM-HG with 2% FBS prior to exposure to IGF-I in DMEM-HG with 0.2% platelet-poor plasma. Forty-eight hours after the addition of IGF-I, the cell number was determined. **, p < 0.01 when the change in cell number in response to IGF-I in LacZ control cells is determined; *, p < 0.05 when the number of cells proliferating after exposure to IGF-I is compared between LacZ and IRS-WT cells. Error bars, mean ± S.E. B, LacZ and IRS-WT cells were grown in 6-well dishes in DMEM-HG containing 10% FBS. After wounding, they were allowed to migrate with or without IGF-I in medium containing 0.2% FBS for 48 h. The total number of cells migrating past the wound line in the predetermined areas was counted. The results shown are the mean ± S.E. of at least three independent experiments. **, p < 0.01 when the change in migrating cells in response to IGF-I in LacZ cells is compared with LacZ cells without IGF-I; *, p < 0.05 when the number of cells migrating in response to IGF-I is compared between LacZ and IRS-WT cells. C, confluent LacZ and IRS-WT cells were serum-starved for 16 h in DMEM-HG and then exposed to IGF-I for the times indicated. The extent of Shc and IRS-1 phosphorylation was determined by immunoprecipitating (IP) p52shc (first panel) or IRS-1 (fourth panel) and then immunoblotting (IB) with an anti-phosphotyrosine (Tyr(P)99 (PY99)) antibody. Similarly, the lysates were immunoprecipitated with anti-Grb2 antibody, and the bottom portion of the blot was used for immunoblotting for p52shc (second panel), whereas the top portion of the same blot was immunoblotted for IRS-1 (fifth panel). An equal amount of protein from cell lysates from the same experiment was used to immunoblot for total Shc (third panel) and total IRS-1 (sixth panel). Similarly, an equal amount of protein from the same cell lysates was immunoblotted using anti-phospho-MAPK (seventh panel). The blots were stripped and reprobed using anti-β-actin antibody (bottom panel). The bar graphs are representative of at least three independent experiments and two independent transductions. Error bars, mean ± S.E. ***, p < 0.001 when the amount of Grb2 associated with Shc at 10 min in response to IGF-I is compared between LacZ and IRS-WT cells; **, p < 0.01 when the amount of phosphorylation of MAPK in response to IGF-I at 10 min is compared between LacZ and IRS-WT cells. D, confluent LacZ- and IRS-WT-expressing cells were serum-starved for 16 h in DMEM-HG and then exposed to IGF-I for 5 min, and the extent of SHPS-1 phosphorylation and SHP-2 association was determined by immunoprecipitating with an anti-SHPS-1 antibody and immunoblotting with Tyr(P)99 (first panel) or with anti-SHP-2 antibody (second panel). The extent of IGF-IR tyrosine phosphorylation was assessed by immunoprecipitating IGF-IR and immuoblotting for phosphotyrosine (fourth panel). Lysates from the same experiments were immunoblotted for total SHPS-1 (third panel) or for total IGF-IR (fifth panel). The bar graph shows the relative increase in SHPS-1 phosphorylation for at least three independent experiments. Error bars, mean ± S.E. ***, p < 0.001 when increase in SHPS-1 phosphorylation in response to IGF-I is compared between LacZ- and IRS-WT-expressing cells.