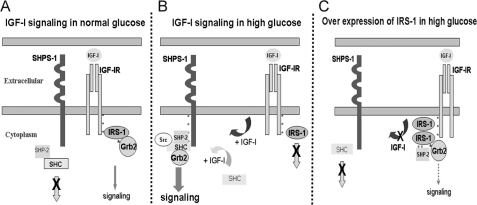
FIGURE 7.
Proposed role of IRS-1 in regulating SHPS-1 phosphorylation under hyperglycemic conditions. A, in VSMCs under normal glucose conditions, in response to IGF-I, competitive binding of IRS-1 to IGF-IR impairs the access of SHPS-1 to IGF-I receptor kinase, thereby decreasing SHPS-1 phosphorylation. Further, IRS-1 sequesters SHP-2, leading to impaired assembly of the signaling complex on SHPS-1. B, in high glucose conditions, reduction in IRS-1 levels allows SHPS-1 improved access to the IGF-I receptor kinase, leading to enhanced phosphorylation of SHPS-1 and subsequent SHP-2 transfer. This facilitates formation of the SHP-2·Src·Shc·Grb-2 signaling complex, which augments phosphatidylinositol 3-kinase and MAPK pathway activation. C, overexpression of IRS-1 decreases SHPS-1 access to the IGF-IR kinase, SHPS-1 phosphorylation, and subsequent complex assembly. The IRS-1 double mutant with impaired binding to IGF-IR and SHP-2 allows increased SHPS-1 access to IGF-IR kinase, thus increasing SHPS-1 phosphorylation and SHP-2 transfer, thereby leading to increased phosphorylation of MAPK and cellular proliferation in response to IGF-I.