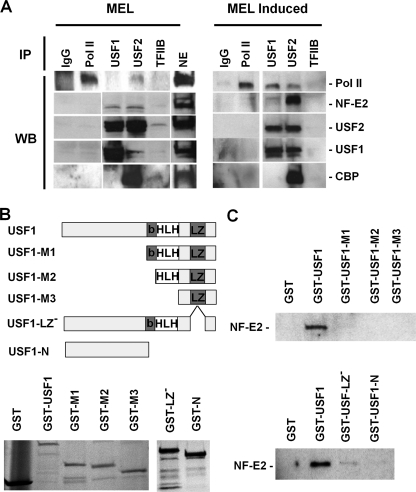
FIGURE 4.
Interactions of USF1 and USF2 with NF-E2 (p45) and Pol II during differentiation of MEL cells. A, co-immunoprecipitation experiments were performed by first subjecting nuclear extracts from MEL cells incubated with or without 1.5% DMSO for 3 days to immunoprecipitation with antibodies specific for IgG, Pol II (N-20), USF1, USF2, and TFIIB. The immunoprecipitated material was electrophoresed using 4–20% Ready Gels (Bio-Rad) and transferred to nitrocellulose membranes. The nitrocellulose membranes were incubated with antibodies against Pol II, NF-E2 (p45), USF1, USF2, and CBP, as indicated, and subjected to ECL Plus chemiluminescence (Amersham Biosciences). B, generation and expression of USF1/GST fusion proteins in E. coli. The cDNA encoding full-length or truncated USF1 was ligated into the pGEX-5X-1 vector. Fusion proteins were expressed in and purified from E. coli and analyzed by SDS-PAGE. The following USF1-derived proteins were purified: full-length USF1 protein (USF1); deletion of the N-terminal half (USF-M1); deletion of the N terminus and the basic region (USF1-M2); deletion of the N terminus, the basic region, and the helix-loop-helix domain (M3); deletion of the leucine zipper (USF-1 LZ−); and deletion of the C terminus as well as the basic region, the HLH domain, and the LZ domain (USF1-N). C, interaction of USF1 with NF-E2. Equal amounts of GST fusion proteins were incubated with His-tagged NF-E2. After washing, proteins were eluted from the GST-beads, electrophoresed using SDS-PAGE, and subjected to Western blotting analysis using NF-E2 (p45)-specific antibodies. WB, Western blot.