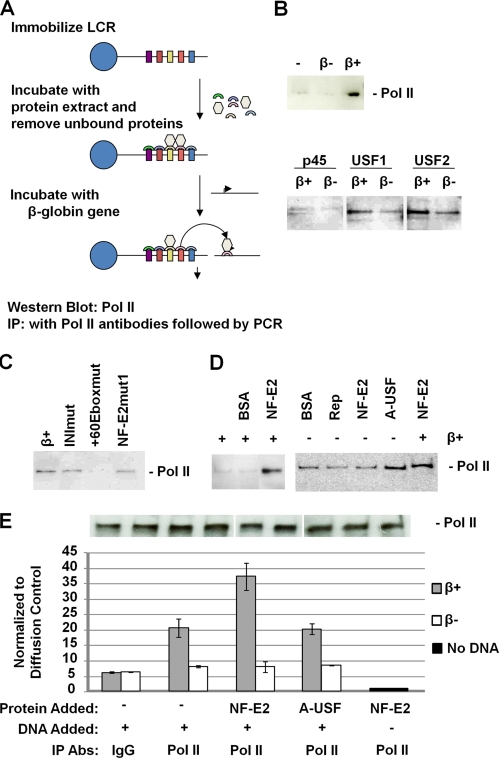
FIGURE 5.
USF and NF-E2 regulate the recruitment and dissociation of Pol II to and from immobilized LCR templates. A, scheme of the experimental strategy. A linearized and biotinylated plasmid containing the human β-globin LCR was immobilized on streptavidin-coated magnetic beads as described by Vieira et al. (45). The LCR was then incubated with whole cell extracts from MEL cells. Unbound material was removed, and the LCR-protein complex was washed several times and incubated with different DNA templates in the presence or absence of recombinant NF-E2 (p45) tethered to MafG (16, 42) or dominant negative USF (A-USF) (13). Proteins that dissociate from the LCR after the incubation step were analyzed using Western blotting analysis. Transfer of proteins to the β-globin gene promoter was analyzed by immunoprecipitation (IP) followed by quantitative PCR. B, β-globin promoter-mediated dissociation of Pol II, NF-E2 (p45), USF1, and USF2, from the LCR in the presence of a plasmid containing the β-globin gene with (β+) or without (β−) its promoter. Proteins and DNA were removed from the immobilized LCR after incubation for 30 min at 30 °C. C, analysis of the effect of β-globin promoter mutations on the dissociation of Pol II. DNA plasmids containing the wild-type β-globin promoter (β+) or the promoter with mutations in the initiator (INImut), the +60 E-box (+60Eboxmut), or the partial MARE sequence (NF-E2mut1) were incubated for 30 min with the immobilized LCR-protein complex in the presence of recombinant NF-E2. Dissociated proteins were removed and analyzed by Western blotting experiments using an antibody specific for Pol II. D, effect of NF-E2 (p45) and A-USF on the dissociation of Pol II from the immobilized LCR. The immobilized LCR-protein complex was incubated for 30 min with BSA, AAV Rep (Rep), NF-E2, or A-USF in the absence or presence of a plasmid containing the wild-type β-globin gene promoter (β+). Dissociated proteins were removed from the LCR and analyzed by Western blotting experiments using an antibody specific for Pol II. E, quantitative PCR analysis of Pol II transfer from immobilized LCR templates to the β-globin gene promoter. Immobilized LCR-protein complexes were incubated with or without a plasmid containing the β-globin gene with (β+) or without (β−) its promoter region for 30 min at 30 °C. Unbound material was subjected to immunoprecipitation using IgG- or Pol II-specific antibodies. The DNA was isolated from the precipitate and subjected to quantitative real-time PCR using primers specific for the β-globin gene. The experiment was repeated, and the error bars represent S.E. An aliquot was taken from immobilized LCR-protein complexes in each transfer dissociation assay and analyzed by Western blotting using Pol II-specific antibodies (shown above the graph).