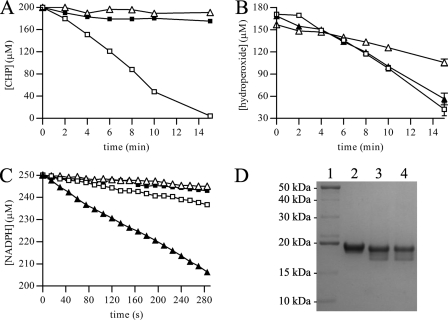
FIGURE 1.
Peroxidase activity of XfPrxQ. A, removal of CHP was determined by DTT-dependent peroxidase assay in the presence of 5 μm XfPrxQ (closed squares), 10 μm XfTsnC (open triangles), and 5 μm XfPrxQ plus 10 μm XfTsnC (open squares). Peroxidase reactions were carried out in a reaction mixture containing 200 μm CHP and 0.5 mm DTT, at 37 °C. B, removal of hydrogen peroxide (open squares), TBHP (open triangles), and CHP (closed triangles) by XfPrxQ in DTT-dependent peroxidase assays. Peroxidase reactions were carried out in a reaction mixture containing 200 μm hydroperoxide, 0.5 mm DTT, 5 μm XfPrxQ, and 7 μm XfTsnC at 37 °C. At the indicated times, the remaining hydroperoxides were quantified in triplicate, and results are the mean ± S.D. (error bars). C, NADPH oxidation coupled to hydrogen peroxide reduction in the presence of 1 μm XfPrxQ (closed squares); 0.8 μm XfTsnC and 0.3 μm XfTrxR (open triangles); 1 μm XfPrxQ, 0.8 μm XfTsnC, and 0.3 μm XfTrxR (closed triangles); and 1 μm XfAhpC, 0.8 μm XfTsnC, and 0.3 μm XfTrxR (open squares). NADPH oxidation was measured at 340 nm in a reaction mixture containing 250 μm NADPH and 500 μm hydrogen peroxide at 37 °C. D, wild-type XfPrxQ was subjected to nonreducing SDS-PAGE under reducing conditions (lane 2), treated with 1 eq of hydrogen peroxide (lane 3) and treated with 5 eq of hydrogen peroxide (lane 4). Lane 1 shows molecular mass standards (BenchMark Protein Ladder, Invitrogen). Reduced XfPrxQ protein was treated with hydrogen peroxide at room temperature for 30 min. Approximately 4 μg of protein was loaded by lane.