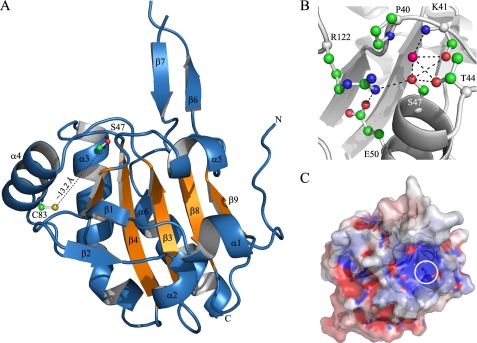
FIGURE 7.
Structural features of the XfPrxQ C47S protein. A, schematic representation of the XfPrxQ C47S structure. The five-stranded mixed β-sheet of the Trx fold is colored in orange. Some elements of secondary structure are labeled, and the N and C termini are indicated. Side chains of Ser-47 and Cys-83 are shown in ball and stick mode. B, interaction network in the active site of XfPrxQ C47S. Residues are represented as balls and sticks, and atoms are colored following the CPK color scheme (carbon, green; oxygen, red; nitrogen, blue; and sulfur, yellow). The magenta ball represents water-290. Hydrogen bonds and salt bridges are indicated by dashed lines. C, XfPrxQ C47S mapped by electrostatic surface potentials (red, negatively charged; blue, positively charged), as calculated with the APBS program (65). The white circle demarcates the surface in the vicinity of the active site.